Question: This is a problem from my thermodynamics course regarding the Gibbs-Duhem equation. Help is appreciated. Derive the Gibbs-Duhem equation as a function of T,P and
This is a problem from my thermodynamics course regarding the Gibbs-Duhem equation. Help is appreciated.
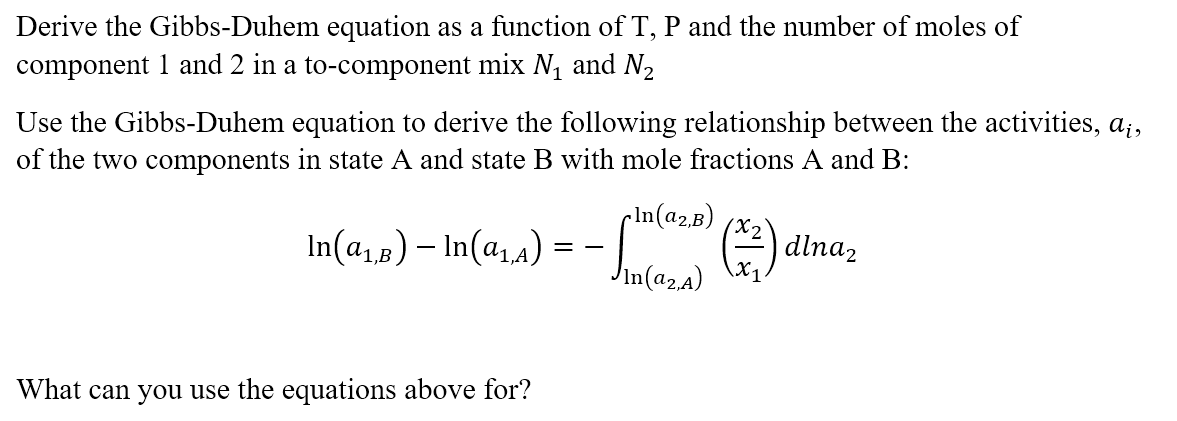
Derive the Gibbs-Duhem equation as a function of T,P and the number of moles of component 1 and 2 in a to-component mixN1 and N2 Use the Gibbs-Duhem equation to derive the following relationship between the activities, of the two components in state A and state B with mole fractions A and B : ln(a1,B)ln(a1,A)=ln(a2,A)ln(a2,B)(x1x2)dlna2 What can you use the equations above for
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


