Question: This is a subjective question, hence you have to write your answer in the fext Fleld given below. Methane gas is compressed in a steady
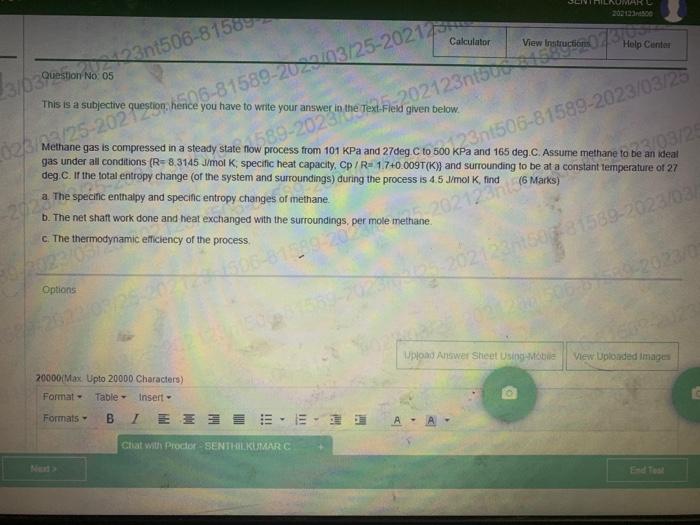
This is a subjective question, hence you have to write your answer in the fext Fleld given below. Methane gas is compressed in a steady state fow process from 101KPa and 27deg.C to 500KPa and 165 deg. C. Assume methane to be an ideal gas under all conditions (R=8.3145 Jimol K; specific heat capacity, Cp/R=1.7+0.009T(K)} and surrounding to be at a conslant temperature of 27 deg. C. If the total entropy change (of the system and surroundings) during the process is 4.5J/molK, find ( 6 Marks) a. The specinc enthalpy and specific entropy changes of methane. b. The net shaft work done and heat exchanged with the surroundings, per mole methane. c. The thermodynamic efficiency of the process
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


