Question: This is based on BioChem covering buffers. please help. Properties of a Buffer The amino acid glycine is often used as the main ingredient of
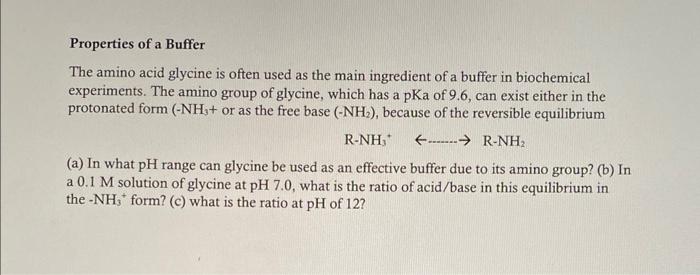
Properties of a Buffer The amino acid glycine is often used as the main ingredient of a buffer in biochemical experiments. The amino group of glycine, which has a pKa of 9.6, can exist either in the protonated form (NH3+ or as the free base (NH2), because of the reversible equilibrium RNH3+..RNH2 (a) In what pH range can glycine be used as an effective buffer due to its amino group? (b) In a 0.1M solution of glycine at pH 7.0, what is the ratio of acid/base in this equilibrium in the NH3+form? (c) what is the ratio at pH of 12
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


