Question: This is chemical engineering and plz answer A and B with picture A process converts toluene (C6H5CH3) and hydrogen to benzene and methane according to
This is chemical engineering and plz answer A and B with picture
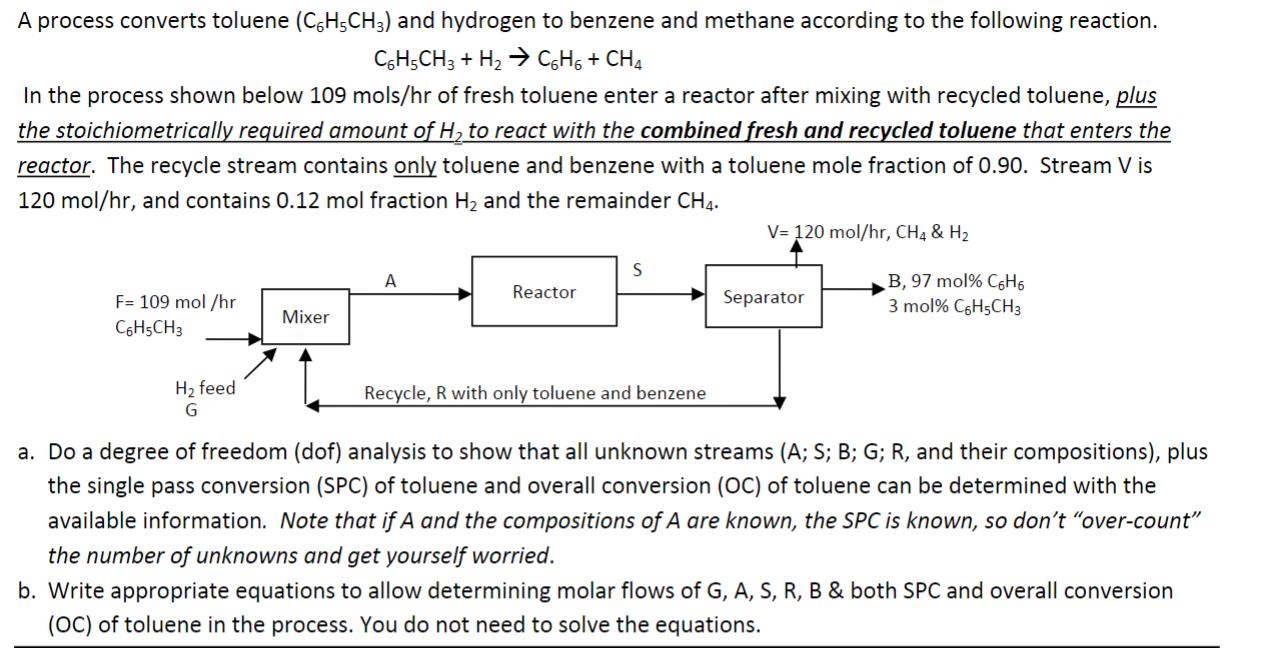
A process converts toluene (C6H5CH3) and hydrogen to benzene and methane according to the following reaction. C6H5CH3+H2C6H6+CH4 In the process shown below 109 mols/hr of fresh toluene enter a reactor after mixing with recycled toluene, plus the stoichiometrically required amount of H2 to react with the combined fresh and recycled toluene that enters the reactor. The recycle stream contains only toluene and benzene with a toluene mole fraction of 0.90. Stream V is 120mol/hr, and contains 0.12 mol fraction H2 and the remainder CH4. a. Do a degree of freedom (dof) analysis to show that all unknown streams (A; S; B; G; R, and their compositions), plus the single pass conversion (SPC) of toluene and overall conversion (OC) of toluene can be determined with the available information. Note that if A and the compositions of A are known, the SPC is known, so don't "over-count" the number of unknowns and get yourself worried. b. Write appropriate equations to allow determining molar flows of G,A,S,R,B \& both SPC and overall conversion (OC) of toluene in the process. You do not need to solve the equations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


