Question: This is Crystal Violet Lab Please help me on 1,2,3,4,5 Here is the information: 1. Was the reaction zero, first, or second order, with respect
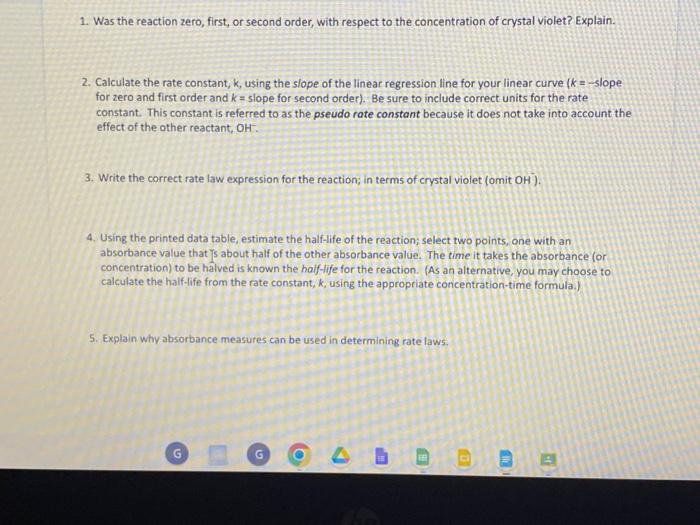
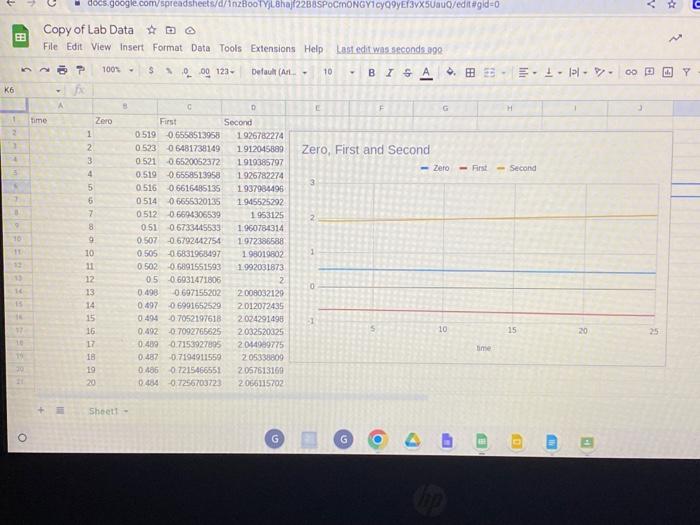

1. Was the reaction zero, first, or second order, with respect to the concentration of crystal violet? Explain. 2. Calculate the rate constant, k, using the slope of the linear regression line for your linear curve (k= slope for zero and first order and k= slope for second order). Be sure to include correct units for the rate constant. This constant is referred to as the pseudo rate constant because it does not take into account the effect of the other reactant, OH. 3. Write the correct rate law expression for the reaction; in terms of crystal violet (omit OH ). 4. Using the printed data table, estimate the half-life of the reaction; select two points, one with an absorbance value that [s about half of the other absorbance value. The time it takes the absorbance for concentration) to be halved is known the half-life for the reaction. (As an alternative, you may choose to calculate the half-life from the rate constant, k, using the appropriate concentration-time formula.) 5. Explain why absorbance measures can be used in determining rate faws. Copy of Lab Data 0 File Edit View Insert Format Data Tools Extensions Help Lasteditwassecondsape: + Shoeti - G ( Procedure Both Colorimeter and Spectrometer Users 1. Obtain and wear goggles. 2. Use a 10mL graduated cylinder to obtain 10.0mL of 0.10MNaOH solution. CAUTION: Sodium hydroxide solution is caustic. Avoid spilling it on your skin or clothing. Use another 10mL graduated cylinder to ASIM Rate Law Determination of the Crystal Violet Reaction student handout, revised 5/2018 Page 1 of 4 obtain 10.0mt of 2.5103M crystal violet solution. CAUTION: Crystal violet is a biological stain. Avold spilling it on your skin or clothing. 3. Prepare a blank by filling a cuvette 3/4 full with distilled water. To correctly use cuvettes, remember: - Wipe the outside of each cuvette with a lint-free tissue. - Handle cuvettes only by the top edge of the ribbed sides. - Dislodge any bubbles by gently tapping the cuvette on a hard surface. - Always position the cuvette so the light passes through the clear sides. 1. Was the reaction zero, first, or second order, with respect to the concentration of crystal violet? Explain. 2. Calculate the rate constant, k, using the slope of the linear regression line for your linear curve (k= slope for zero and first order and k= slope for second order). Be sure to include correct units for the rate constant. This constant is referred to as the pseudo rate constant because it does not take into account the effect of the other reactant, OH. 3. Write the correct rate law expression for the reaction; in terms of crystal violet (omit OH ). 4. Using the printed data table, estimate the half-life of the reaction; select two points, one with an absorbance value that [s about half of the other absorbance value. The time it takes the absorbance for concentration) to be halved is known the half-life for the reaction. (As an alternative, you may choose to calculate the half-life from the rate constant, k, using the appropriate concentration-time formula.) 5. Explain why absorbance measures can be used in determining rate faws. Copy of Lab Data 0 File Edit View Insert Format Data Tools Extensions Help Lasteditwassecondsape: + Shoeti - G ( Procedure Both Colorimeter and Spectrometer Users 1. Obtain and wear goggles. 2. Use a 10mL graduated cylinder to obtain 10.0mL of 0.10MNaOH solution. CAUTION: Sodium hydroxide solution is caustic. Avoid spilling it on your skin or clothing. Use another 10mL graduated cylinder to ASIM Rate Law Determination of the Crystal Violet Reaction student handout, revised 5/2018 Page 1 of 4 obtain 10.0mt of 2.5103M crystal violet solution. CAUTION: Crystal violet is a biological stain. Avold spilling it on your skin or clothing. 3. Prepare a blank by filling a cuvette 3/4 full with distilled water. To correctly use cuvettes, remember: - Wipe the outside of each cuvette with a lint-free tissue. - Handle cuvettes only by the top edge of the ribbed sides. - Dislodge any bubbles by gently tapping the cuvette on a hard surface. - Always position the cuvette so the light passes through the clear sides
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


