Question: This is for a basic programming class that starts off with Excel. I have to create an excel spreadsheet that calcuates the compressibility factor of
This is for a basic programming class that starts off with Excel.
I have to create an excel spreadsheet that calcuates the compressibility factor of an ideal gas, known as (z). CO2, N2, and Cl2, assuming vanderwaals behavior. It says the easiest way to solve this is to use a feature of excel called circular reference, because use of the spreadsheet "solver" factor isnt accurate enough. The equation for compressibility is z=(PVRT). There are few set parameters for this problem: n (# of moles) = 1. R = 8.314 J/mol-k, and T = 300 K.
The pressures are what change: I need to find Z for all of the following pressures: (All in atm): 0, 75, 150, 225, 300, 375, 450, 525, 600, and 675.
The compressibility of an ideal gas at P = 0 is 1. At P = 0, all gasses behave like ideal gases.
I was told to rewrite the PV=nRT equation as the following. V = ((V^2)nRT)/((V^2)P = a(n^2)) +nb
The constants (a) and (b) for each gas are listed below in a screenshot of one of my excel tables. n =1 mole, T = 300K, and R = 8.314 J/mol-K
It was also made explicitly clear to me that I need to solve this problem using the circular reference feature of the excel spreadsheet, and I absolutely cannot figure out how to use it or how it works. I've read through the text repeatedly, and tried to use youtube but I've run headlong into a wall. Thanks so much for you help!
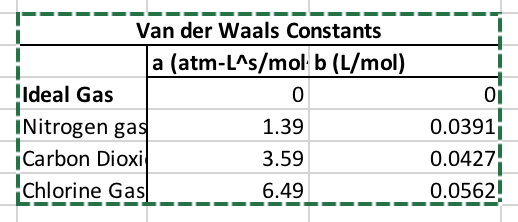
Van der Waals Constants a (atm-LAs/mol b (L/mol) Ideal Gas INitrogen gas Carbon Dioxi Chlorine Gasl 01 0 1.39 3.59 6.49 0.0391 0.0427 -o---0.0562
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


