Question: This is for a Python programming class, try if you can to use the simplest code to understand possible even if it may not be
This is for a Python programming class, try if you can to use the simplest code to understand possible even if it may not be the most efficient.
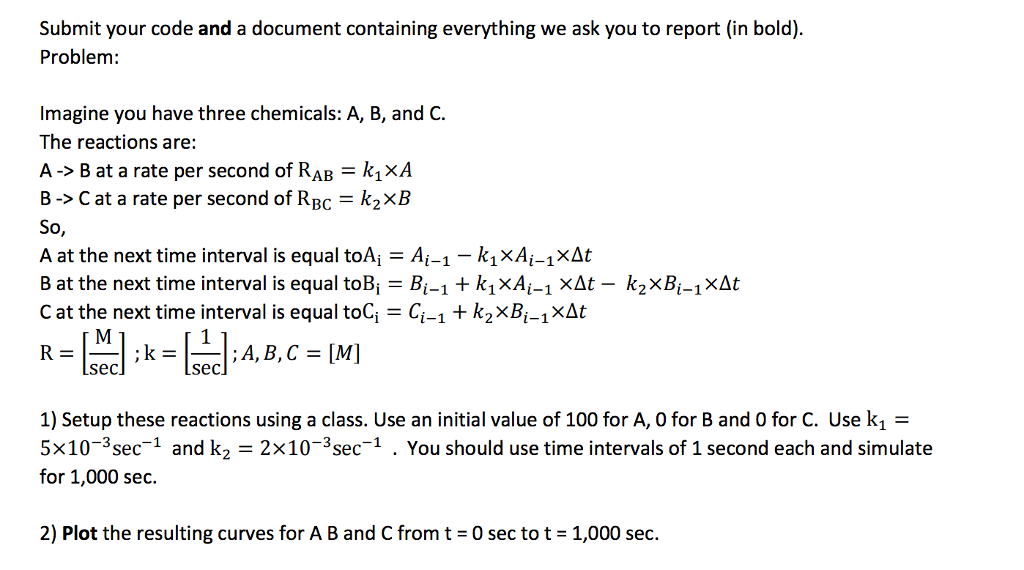
Submit your code and a document containing everything we ask you to report (in bold) Problem: Imagine you have three chemicals: A, B, and C The reactions are: A B at a rate per second of RAB kixA B-> Cat a rate per second of R BC ks, XB So A at the next time interval is equal toA i-1 i-1 xAt B at the next time interval is equal toB i-1 i-1 XAt k2x i-1 XAt C at the next time interval is equal toC i-1 XAt M A, B, C MI Sec Sec 1) Setup these reactions using a class. Use an initial value of 100 for A, 0 for B and 0 for C. Use k1 5x10 sec 1 and k 2x 10 sec You should use time intervals of 1 second each and simulate for 1,000 sec 2) Plot the resulting curves for ABand C from t 30 sec to t -1,000 sec
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


