Question: This is for a thermodynamis class that is asking for quantitaive answers, thank you! 2. A new steam reformer creates both H and monatomic hydrogen.
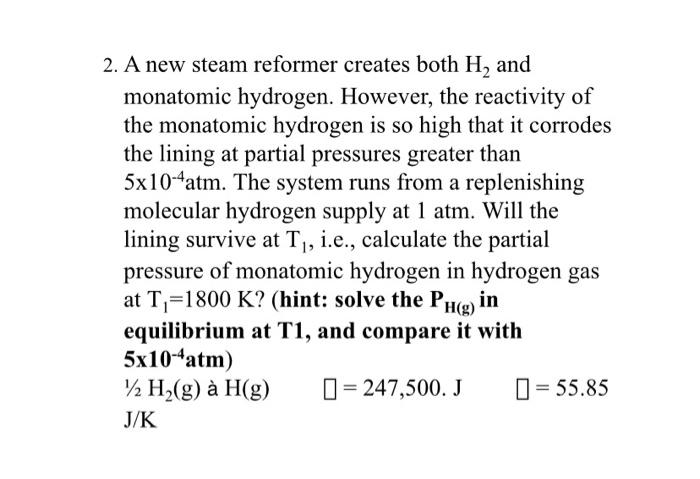
2. A new steam reformer creates both H and monatomic hydrogen. However, the reactivity of the monatomic hydrogen is so high that it corrodes the lining at partial pressures greater than 5x10-4atm. The system runs from a replenishing molecular hydrogen supply at 1 atm. Will the lining survive at T, i.e., calculate the partial pressure of monatomic hydrogen in hydrogen gas at T=1800 K? (hint: solve the PH(g) in equilibrium at T1, and compare it with 5x10-4atm) /2 H(g) H(g) = 247,500. J = 55.85 J/K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


