Question: This is from a past year and I am having difficulty with the answer. For question B, please draw your answer in the same format
This is from a past year and I am having difficulty with the answer. For question B, please draw your answer in the same format as the diagram so I know how the question is supposed to be answered, thank you!

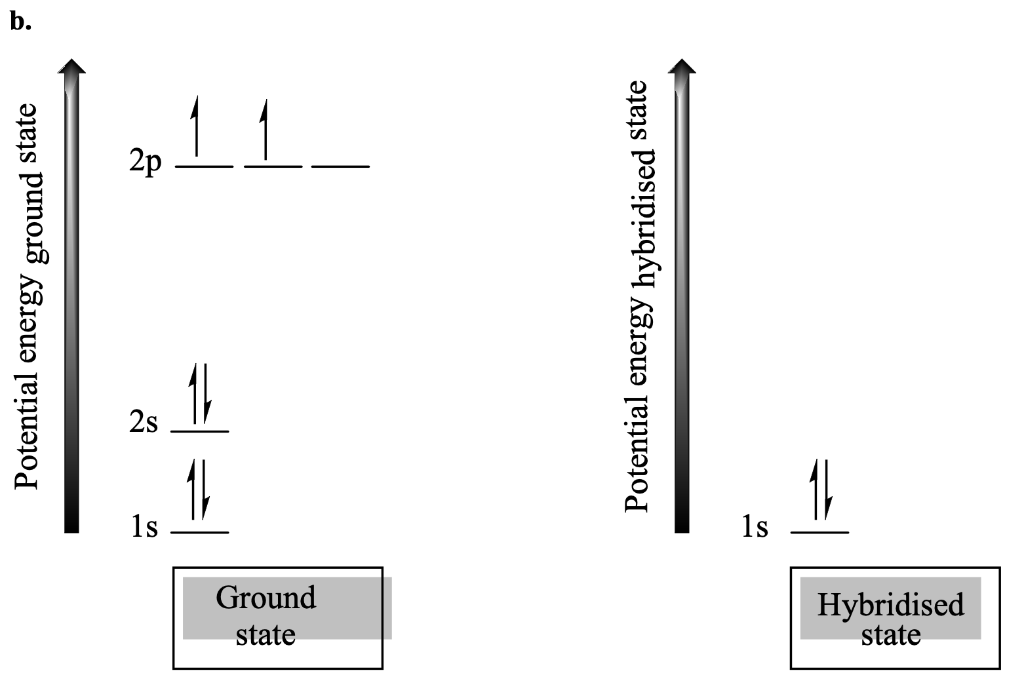
QUESTION 52-WRITE YOUR ANSWER IN THE SPACE PROVIDED [5 marks] Each carbon in ethene (C2H2) is hybridised. a. Draw the Lewis diagram for ethene and indicate the hybridisation state for the carbons. [2 marks] b. Draw the orbital diagram for the hybridised carbons in ethene, clearly showing the hybrid carbon orbitals, as well as the electrons that they hold. [3 marks] For reference, the ground state orbitals for an unhybridised carbon have been included. on the left. Hint: You need only consider what happens to the electrons in the n=2 orbitals in the hybridised state. 1 state 2p Potential energy ground state Potential energy hybridised 2s 11. 11. 11. 1s 1s Ground state Hybridised state
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


