Question: This is how my question was given to me , A mixture of propane and n - hexane at 4 3 . 5 psi is
This is how my question was given to me
A mixture of propane and nhexane at psi is to be separated.
a Generate xyplot for propane for this mixture at the given pressure. Use data in the DePriester charts, DDB or any other source.
b If of the mol feed is vaporized, what are the liquid and vapor mole fractions. Also, what is the drum temperature?
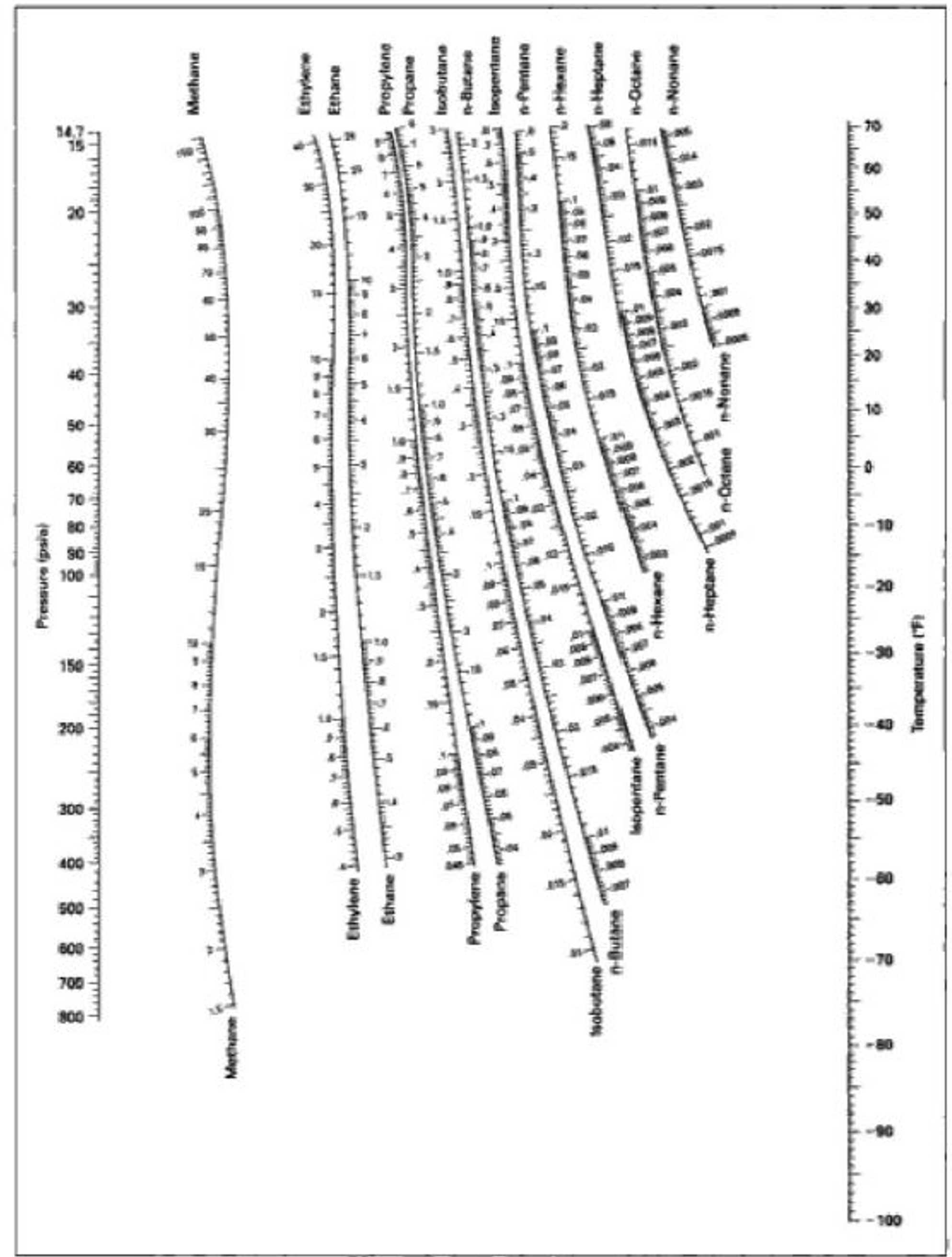
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


