Question: This is my data Please help me and show me your work. Thank you Data Analysis: Do the following calculations for each determination and record

This is my data
Please help me and show me your work. Thank you
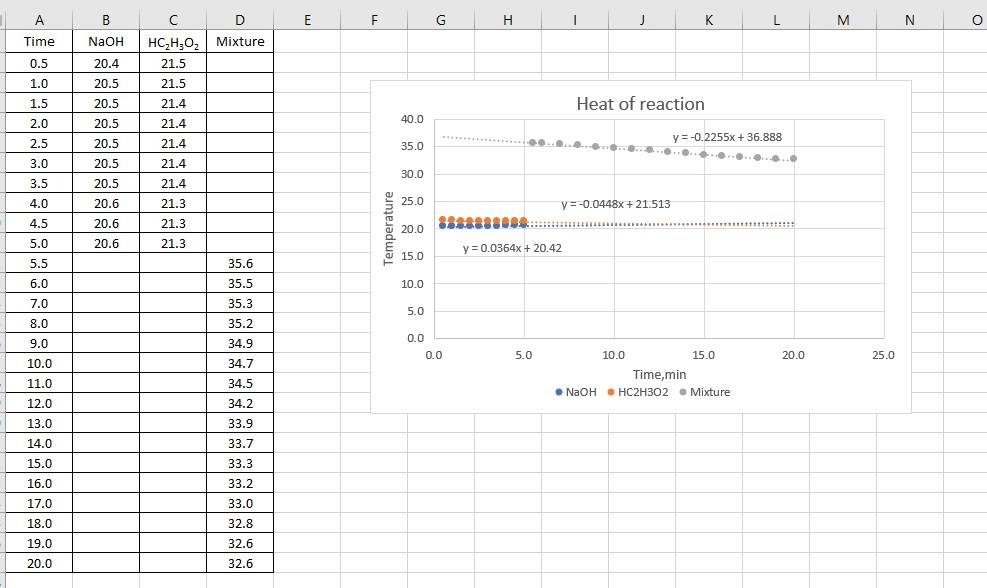
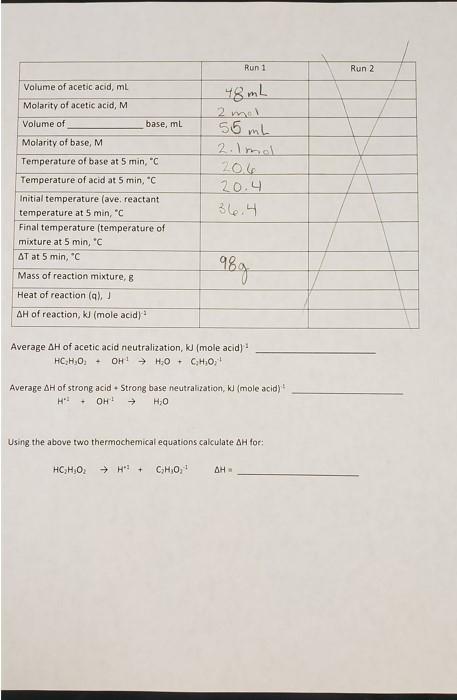
Data Analysis: Do the following calculations for each determination and record you results on back of your data sheet. 1.) Plot the time-temperature data for each determination as recorded on the front side of your data sheet. Use an appropriate spreadsheet program. Use trend lines and their equations to calculate the temperature of each component at 5 minutes. 2.) The initial temperature of the acid and base is the 5 minute temperature respectively. Average both values and record as Tinitial. Tfinal is the temperature at 5 minutes of the reaction mixture. AT = Tfinal - Tinitial 3.) Calculate the heat transferred, q, when the acid reacted with the base. Assume that the density of the reaction mixture is 1.04 g/mL and that the volumes are additive. The specific heat capacity of the reaction mixture is 3.89 J4g deg). q=__(specific heat capacity) (mass of solution) (AT) 4.) Calculate AH.reaction the molar enthalpy of reaction by AHreaction solution / (moles of H+1 reacting) 5.) Repeat the calculations for the second determination. 6.) Calculate and record the average molar enthalpy of reaction for your two determinations. 7.) Transfer the average enthalpy of neutralization for a strong acid and strong base from Thermochemistry I to your data sheet. Using this information with the average enthalpy of neutralization for acetic acid and a strong base you determined today, to determine the heat of dissociation for acetic acid. A B B E F G H 1 J K L M N O Time NaOH D HC,H,O, Mixture 21.5 20.4 0.5 1.0 20.5 21.5 21.4 1.5 20.5 Heat of reaction 2.0 20.5 40.0 20.5 y=-0.2255x + 36.888 35.0 21.4 21.4 21.4 21.4 2.5 3.0 3.5 ......... 20.5 20.5 30.0 4.0 20.6 21.3 25.0 y = -0.0448x + 21.513 y 4.5 21.3 20.6 20.6 Temperature 20.0 5.0 21.3 y = 0.0364x + 20.42 15.0 5.5 35.6 6.0 35.5 10.0 7.0 35.3 5.0 8.0 35.2 34.9 0.0 0.0 5.0 20.0 25.0 9.0 10.0 11.0 34.7 10.0 15.0 Time,min NaOH HC2H302 - Mixture 34.5 12.0 34.2 33.9 13.0 14.0 15.0 16.0 33.7 33.3 33.2 33.0 17.0 18.0 19.0 32.8 32.6 32.6 20.0 Run 1 Run 2 Volume of acetic acid, ml Molarity of acetic acid, M Volume of base, ml Molarity of base, M 48 mL 2 mol 55 mh 2.1 mol 206 20.4 36.4 Temperature of base at 5 min, "C Temperature of acid at 5 min, "C Initial temperature (ave, reactant temperature at 5 min, C Final temperature (temperature of mixture at 5 min, 'C AT at 5 min, "C 98g Mass of reaction mixture, Heat of reaction (9), AH of reaction, I (mole acid) Average AH of acetic acid neutralization, KJ (mole acid) HLH 01 - OHHO+ CH,O, Average AH of strong acid . Strong base neutralization, kl (mole acid) HP OH! HO - Using the above two thermochemical equations calculate AH for: HC H02 CHO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


