Question: this is one question with three parts please solve all parts thankyou! An electric heater supplies 16.9 joules of energy to 24.8g sample of H2O

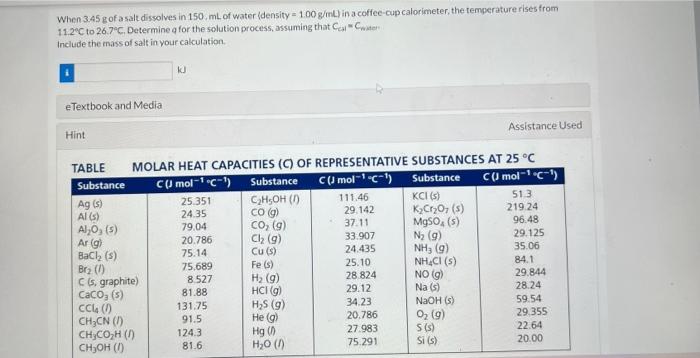

An electric heater supplies 16.9 joules of energy to 24.8g sample of H2O originally at 35.3C. Compute the final temperature (in C ). eTextbook and Media Hint Assistance Used The molar heat capacity of sample of H2O is 75.291Jmol1K1. Attempts: 2 of 4 used When 3.45g of a sale dissolves in 150,mL of water (density =1.00g/mL ) in a coffec-cup calorimeter, the temperature rises from 11.2C to 26.7C. Determine q for the solution process, assuming that Ca1=Cmiter Include the mass of salt in your calculation. eTextbook and Media A block of copper weighing 16.3g is heated to 78.4C in boiling ethanol. It is then dropped into 33.9g of ethanol initially at 10.5C. Find the final temperature of copper + ethanol in C. C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


