Question: this is part A ( iodine mix with an unknown metal A) part B use the same ratio but mix CrO4 with unknown metal B)
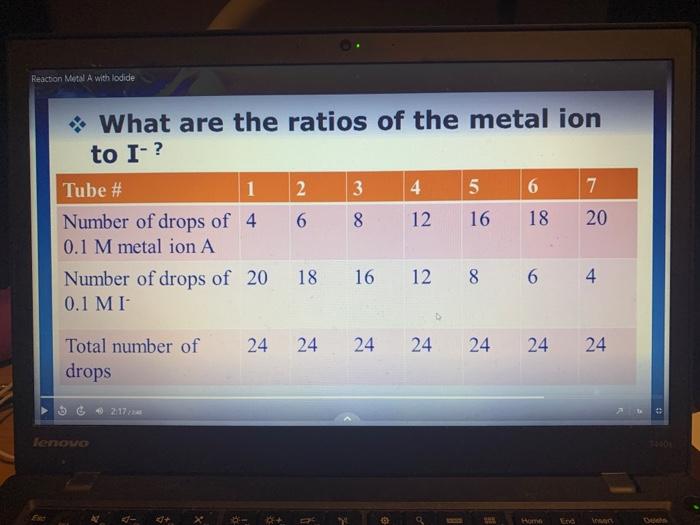 this is part A ( iodine mix with an unknown metal A)
this is part A ( iodine mix with an unknown metal A) Question 4 - Write text or insert photo of your answer Based on the results of your experiment, write separate balanced net ionic equations for the ions reacting in Part A and Part B of the experiment. (2 marks) What are the ratios of the metal ion to I? Question 4 - Write text or insert photo of your answer Based on the results of your experiment, write separate balanced net ionic equations for the ions reacting in Part A and Part B of the experiment. (2 marks) What are the ratios of the metal ion to
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


