Question: Suppose a piece of silver jewelry contains 5.11x1022 atoms of silver (Ag). Part A How many dozens of silver atoms are in the piece
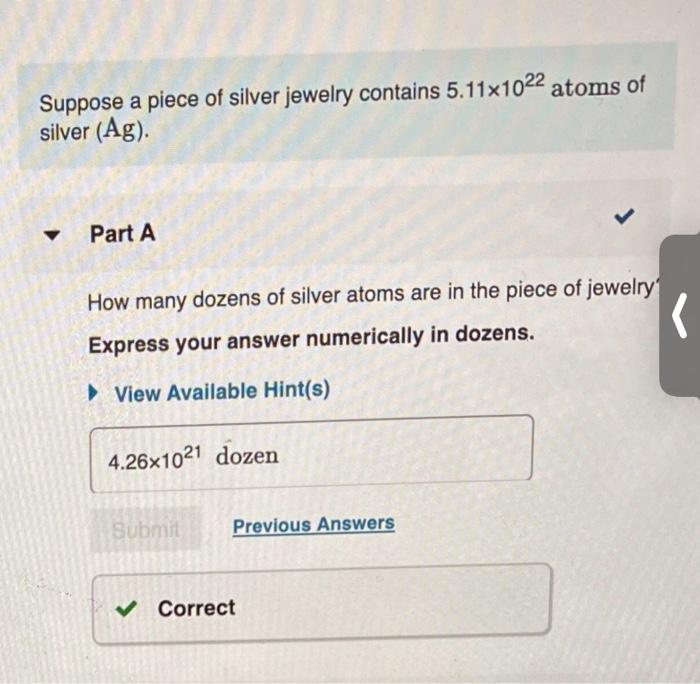
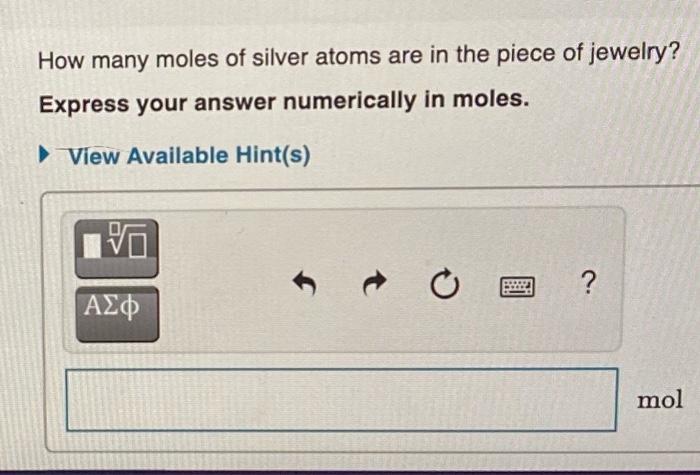
Suppose a piece of silver jewelry contains 5.11x1022 atoms of silver (Ag). Part A How many dozens of silver atoms are in the piece of jewelry Express your answer numerically in dozens. ( View Available Hint(s) 4.261021 dozen Submit Previous Answers V Correct How many moles of silver atoms are in the piece of jewelry? Express your answer numerically in moles. View Available Hint(s) | ? mol
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


