Question: This is the question (the flowchart) Then this is the material (below) Flow Chart for group D Separation. Mg2+, Ni2+, Cu2+, Ca2+, Zn2+ 6 M
This is the question (the flowchart)
Then this is the material (below)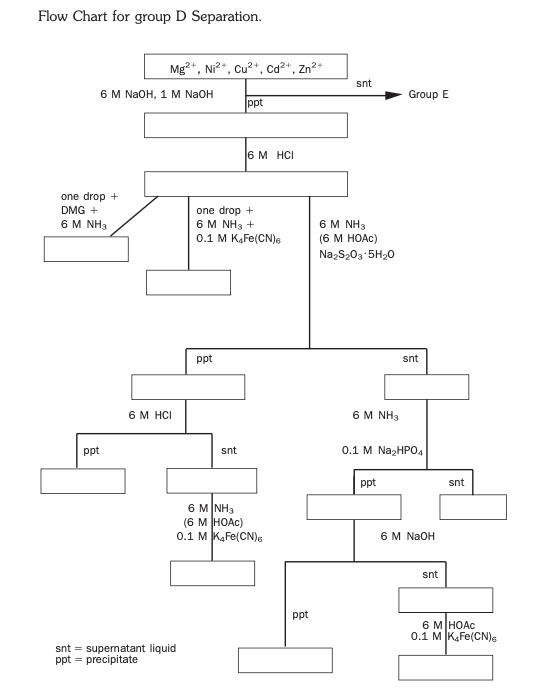






Flow Chart for group D Separation. Mg2+, Ni2+, Cu2+, Ca2+, Zn2+ 6 M NaOH, 1 M NaOH snt Group E ppt 6 M HCI one drop + DMG + 6 M NH3 one drop + 6 M NH3 + 0.1 M K Fe(CN) 6 M NH3 (6 M HOC) Na2S203-5H20 ppt snt 6 M HCI 6 M NH3 ppt snt 0.1 M Na2HPO4 ppt snt 6 M NH3 (6 M HOAC) 0.1 MK4Fe(CN) 6 M NaOH snt ppt 6 MHOAC 0.1 MK4Fe(CN) snt = supematant liquid ppt = precipitate Confirmation of Group D Cations Nickel lon Nickel is confirmed directly from the dissolved hydroxide residue. A drop of this solution is mixed with a drop of dimethylglyoxime solution. The mixture is made basic with aqueous ammonia. A cherry red nickel dimethylglyoxime complex (ab- breviated DMGH, the H signifying that one acidic hydrogen is easily removed) precipitate forms if nickel is present. Nickel forms the only highly colored pre- cipitate with dimethylglyoximate ions. Note that the solution must be basic in or- der for the precipitate to form after the DMGH deprotonates to DMG. Ni(H20)?" (aq) + 6 NH3(aq) Ni(NH3)+ (aq) + 6H2O (8) Ni(NH3)3+ (aa) + 2 DMGHN:(DMG)29) + 4 NH3(aa) + 2 NH (aa) (9) Copper (11) lon Copper ion is also confirmed directly from the dissolved hydroxide residue. The confirmation test is the formation of a maroon precipitate of copper hexa- cyanoferrate, CuzFe(CN)6, from slightly acidic solution. In the absence of Cu2+, a white, yellow, or pale green precipitate may be observed, as ferrocyanide forms precipitates with most of the Group D cations. 2 Cu(NH3)3 (a) + Fe(CN), (aa)+Cu2Fe(CN)69) + 8 NH (a) (10) The strongly acidic solution is treated with aqueous ammonia until it is only slightly acidic. The purpose of this is to avoid the addition of hexacyanofer rate to a strongly acidic solution, which could result in the formation of hydrogen cyanide (HCN), a very toxic gas. By reducing the acidity of the solution, this possibility is avoided. Cadmium Cadmium is particularly difficult to identify, as it must be isolated from virtu- ally all other cations. This is due to the fact that most cadmium compounds are white to pale yellow, and their presence is easily masked by the presence of even traces of copper and nickel cations that form highly colored com- pounds. In addition, zinc compounds are also mostly white or pale yellow. Cadmium can be separated from nickel, zinc, and copper based on the sol- ubilities of their sulfides. Cadmium sulfide is considerably more soluble than either copper or nickel sulfide. Zinc sulfide is much more soluble and under the conditions used, it does not precipitate. Magnesium does not form a sul- fide. Copper and nickel sulfides are black, whereas cadmium sulfide is yellow. (For an unknown, if the sulfide precipitate is yellow, no further test for cad- mium is necessary.) In hot, weakly acidic solution, sodium thiosulfate disproportionates to form sulfate and sulfide ions. This is the source of sulfide ions to precipitate the Cu2+, Ni2+, and Cd2+ ions, leaving Mg2+ and Zn2+ in solution. The Ni2+ ions may not completely precipitate as the sulfide in weakly acidic solution, so some Ni2+ may remain dissolved, along with the Zn2+ and Mg2+. This su- pernatant is used later to test for the presence of Zn2+ and Mg2+ After centrifuging, the precipitate is treated briefly with HCl, then cen- trifuged. The HCI dissolves the Cds, leaving NiS and Cus as solids. This treat- ment must be done quickly and at room temperatureif the exposure to HCI is too long or at an elevated temperature, CuS and Nis may begin to dissolve, negating the separation. The remaining NiS and CuS solids may be discarded and the supernatant liquid is tested for the presence of Cd2+. The diagnostic test for Cd2+ is the precipitation of white cadmium hexacyanoferrate in weakly acidic solution + H2O + + SOX (aq+ Haq) (11) 2 dag) + Fe(CN) (aq) Cd Fe(CN)6(9) (12) The supernatant containing the Cd2+ is initially very acidic from the HCl. As in the identification of Cu2+, the solution is made weakly acidic by adding am- monia, and then is treated with hexacyanoferrate. (ag) Magnesium The identification of magnesium is accomplished using the supernatant re- maining after the precipitation of Cu2+, Ni2+, and Cd2+ as sulfides. This su- pernatant contains Zn2+, Mg2+, and probably some Ni2+ (the presence of Ni2+ will give a blue color to the solution). The solution is made basic with aqueous ammonia, and disodium hydrogen phosphate, Na2HPO4, is added. A white precipitate of zinc phosphate, Zn3(PO4)2, and magnesium ammonium phosphate, MgNH4PO4, forms. Any other Group D cations present at this point remain dissolved as their phosphates are soluble in ammonia. Ha + NH3(aq) NH(aq) (13) Mgle + NH4 (q) + HPO (aq) MgNH PO4() + Haq) (14) 3 Zn + 2 HPO2 (aq) + 2 NH3 +Zn3(PO4)2x) + 2 NH.(a) (15) If any Ni2+ is present, it will undergo reaction according to Reaction (8), yield- ing a blue nickel hexammine complex. After being centrifuged, the supernatant is discarded and the precipitate is treated with NaOH. Any zinc phosphate will dissolve to form the colorless tetrahydroxozincate(II) species, Zn(OH). Any remaining white precipitate is magnesium ammonium phosphate that is insoluble in base solution, proving the presence of magnesium. Zn3(PO4)269) + 12 OHaq) +3 Zn(OH) (aq) + 2 PO (aq) (16) + Identification of Zinc The basic supernatant is tested for the presence of zinc by making the solution slightly acidic with aqueous acetic acid (CH3COOH). A white precipitate of Zn,Fe(CN)6 forming on the addition of K4Fe(CN)6 proves the presence of Zn2+. L INTRODUCTION The Group D cations are characterized by having hydroxides, oxides, and ox- alates that are soluble in ammonia solution. Their oxides are insoluble in strongly basic solutions that do not contain ammonia. These cations are magnesium, Mg2+; nickel(II), Ni2+; copper(II), Cu+; zinc(II), Zn2+; and cadmium(II), Cd2+ Separation of the Group D Cations NOTE: The redox reactions below are not balanced. This is left as an exercise (see Pre-Laboratory Question 1). Metal cations dissolved in aqueous solution are usually present as aquo com- plexes, that is, some fixed number of H2O molecules are formally bonded to the metal (e.g., Ni(H20)2+). Many transition metal aquo complexes are colored, while those of the s-block metals are colorless. Of the Group D cations, only Ni2+ and Cu2+ have colored aquo complexes (green and blue, respectively). In the fol- lowing reactions, the starting form of the cations is written without the H20. The solution containing the Group D cations is first treated to remove any Group A cations as insoluble chlorides. The Group B cations are then pre- cipitated as oxides and hydroxides with the addition of aqueous ammonia. The Group C cations are then precipitated by adding ammonium oxalate solution. Upon addition of ammonia, four of the Group D cations form ammonia complexes. Of these, the copper complex is royal blue, the nickel complex is medium blue, and the cadmium and zinc complexes are colorless. These complexes are very stable, thus the oxalates do not precipitate in ammonia solution, even though they are relatively insoluble in deionized water. The mag- nesium ion does not form a complex with ammonia, but its hydroxide and oxalate are more soluble and do not precipitate. Thus, the Group D cations remain in the supernatant liquid. Cukai + 4 NH3(aq) + Cu(NH3)** (aq) (1) Cd2+ 4 NH3(aq) Cd(NH3) & (aq) (2) Ni(NH3) & ad (3) Znam + 4 NH3(aq) Zn(NH3)+ (aq) (4) Nilag + 6 NH3(aq) Magnesium The identification of magnesium is accomplished using the supernatant re- maining after the precipitation of Cu2+, Ni2+, and Cd2+ as sulfides. This su- pernatant contains Zn2+, Mg2+, and probably some Ni2+ (the presence of Ni2+ will give a blue color to the solution). The solution is made basic with aqueous ammonia, and disodium hydrogen phosphate, Na2HPO4, is added. A white precipitate of zinc phosphate, Zn3(PO4)2, and magnesium ammonium phosphate, MgNH4PO4, forms. Any other Group D cations present at this point remain dissolved as their phosphates are soluble in ammonia. Ha + NH3(aq) NH(aq) (13) Mgle + NH4 (q) + HPO (aq) MgNH PO4() + Haq) (14) 3 Zn + 2 HPO2 (aq) + 2 NH3 +Zn3(PO4)2x) + 2 NH.(a) (15) If any Ni2+ is present, it will undergo reaction according to Reaction (8), yield- ing a blue nickel hexammine complex. After being centrifuged, the supernatant is discarded and the precipitate is treated with NaOH. Any zinc phosphate will dissolve to form the colorless tetrahydroxozincate(II) species, Zn(OH). Any remaining white precipitate is magnesium ammonium phosphate that is insoluble in base solution, proving the presence of magnesium. Zn3(PO4)269) + 12 OHaq) +3 Zn(OH) (aq) + 2 PO (aq) (16) + EXPERIMENTAL PROCEDURE It is usually convenient to do Parts A (the known mix- ture) and B (your unknown) simultaneously. Unless oth- erwise indicated, discarded solutions (including washes) should be collected for disposal. Some solutions with spe- cial hazards should be collected separately, according to your instructor's directions. Be sure to record all reagents added and all observations (including before and after centrifuging) on the data sheets. PART A: PRECIPITATION OF (NOTE: If you are analyzing a solution as part of a continuing ex- THE GROUP D CATIONS IN A periment, use the supernatant left from separating the Group C KNOWN MIXTURE cations and begin at the Precipitation of Group D cations section below.) In a clean 10 x 75 mm test tube, obtain 10 drops of a solution that is approximately 0.1 M in each of the four Group D cations. Add 4 drops of 6 M HCI (Caution: corrosive). Any precipitate here contains Group A cations and should be centrifuged and removed. Add 6 M ammonia (Caution: irritant) dropwise to the supernatant, with stirring and counting the drops, until the solution tests neutral to pH paper. Add an equal number of drops of 6 M ammonia in excess and stir. Any precipitate here contains Group B cations and should be centrifuged and removed . Add 5 drops of 0.5 M ammonium oxalate solution, stir thoroughly, and centrifuge for 2 minutes. Any precipitate present will contain cations from Group C. The supernatant contains the Group D and Ecations. Record all observations. Precipitation of the Group D Cations Decant the supernatant liquid containing the Group D and Ecations into a clean crucible. Place the lid on the crucible, slightly ajar, and heat the pre- cipitate to dryness over a low flame. . Cool the crucible for 5 minutes, and then add 6 drops of concentrated ni- tric acid (Caution: corrosive and oxidizer), washing the inside of the crucible. Replace the lid and heat to dryness once again (HOOD). . Cool the crucible for 5 minutes, repeat the addition of concentrated nitric acid and heat to dryness (HOOD). . Cool the crucible for 5 minutes, and dissolve the residue in 5 drops of 6 M HCI. Using a clean Pasteur pipet, transfer the solution to a clean 10 x 75 mm test tube labeled "A." Rinse the crucible with 5 drops of deionized water and add this rinse to the same test tube. Record all observations. Identification of Nickel(II) lon Transfer one drop of solution A to a clean test tube. Add one drop of 1% dimethylglyoxime. Add 6 M NH3 with stirring, until the solution tests basic to litmus paper. Record all observations. Experiment 33 / Separation and Identification of the Group D Cations 441 Identification of Copper(II) lon . Transfer a second drop of solution A to a clean test tube. Add 6 M NH3 until the solution tests only weakly acidic. If the solution becomes basic, use 6 M acetic acid to make it slightly acidic. Add 3-4 drops of 0.1 M K Fe(CN). solution. Record all observations. Separation and Identification of Cadmium(II) lon . Add 6 M NH3 to the remainder of solution A until it tests only weakly acidic. If the solution becomes basic, use 6 M acetic acid to make it slightly acidic. . Add about 200-300 mg of solid sodium thiosulfate, Na2S2O3, and heat for 5 minutes in a boiling water bath. Cool for 1 minute by swirling the test tube in cold tap water . Centrifuge and decant the supernatant into a clean test tube labeled "B" for magnesium analysis. Wash the precipitate with 10 drops of deionized water. Discard the wash. To the precipitate, add 5 drops of 6 M HCl and stir for 10-15 sec. Im- mediately centrifuge for 1 minute, and quickly decant the supernatant into a clean test tube. . Add 6 M NH3 to the liquid, until the solution tests only weakly acidic. If the solution becomes basic, use 6 M acetic acid to make it slightly acidic. Add 5 drops of 0.1 M K4Fe(CN)solution. Record all observations. Identification of Magnesium lon . To solution B, add 6 M NH3 until the solution tests basic to litmus paper (pH 9-10). Add 6-8 drops of 0.1 M Na2HPO4 solution. Let the solution sit several minutes. Centrifuge and discard the supernatant. Wash the precipitate with 10 drops of deionized water. Add 6 drops of 6 M NaOH (Caution: corrosive and toxic) and stir thoroughly. Centrifuge and decant the supernatant into a clean test tube labeled "C." Record all observations. Identification of Zinc lon . To the supernatant in test tube "C," add 6 M acetic acid with stirring until the solution tests weakly acidic to litmus paper (pH 4-6). Add 4 drops of 0.1 M K4Fe(CN)6. Record all observations. PART B: ANALYSIS OF AN UNKNOWN CONTAINING THE GROUP D CATIONS Obtain 10 drops of an unknown solution containing one or more of the Group D cations in a clean centrifuge tube. Repeat the procedures outlined in Part A, using the unknown solution. Report which cations are present in the unknown, along with observations to verify the identification
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


