Question: this is the third time i've asked this question now, but still nobody has answered with an actual explanation on how to solve for this
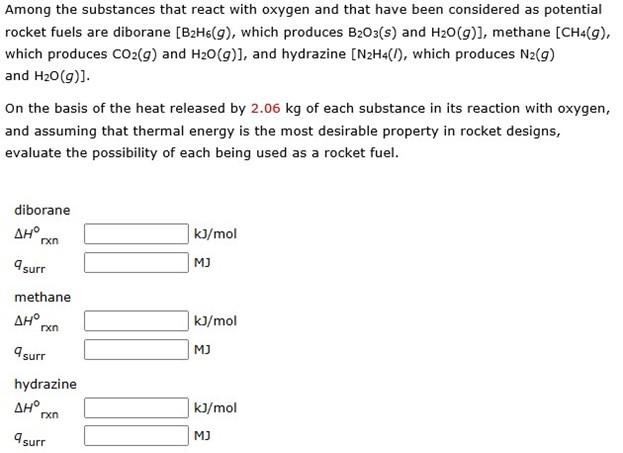
this is the third time i've asked this question now, but still nobody has answered with an actual explanation on how to solve for this problem and i desperately need the help solving it.
Among the substances that react with oxygen and that have been considered as potential rocket fuels are diborane [B2H6(g), which produces B2O3(s) and H2O(g)], methane [CH4(g), which produces CO2(g) and H2O(g)], and hydrazine [N2H4(I), which produces N2(g) and H2O(g)] On the basis of the heat released by 2.06kg of each substance in its reaction with oxygen, and assuming that thermal energy is the most desirable property in rocket designs, evaluate the possibility of each being used as a rocket fuel
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


