Question: This lab uses the Build an W from MI Interactive-e Simulations at University of lColorado Boulder, under the CC-BY 4i} license- Simulation Link: hwcoloradoedufsimsfhtll!'ldanatom'iatestfbuildanatom en.html

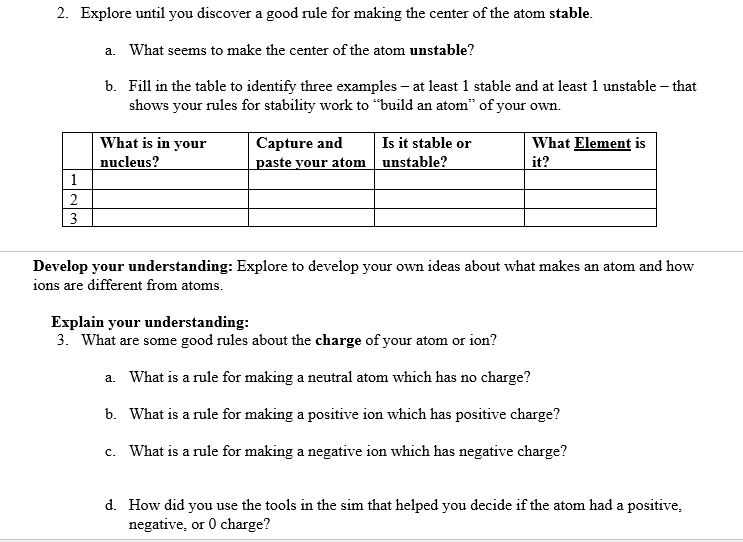
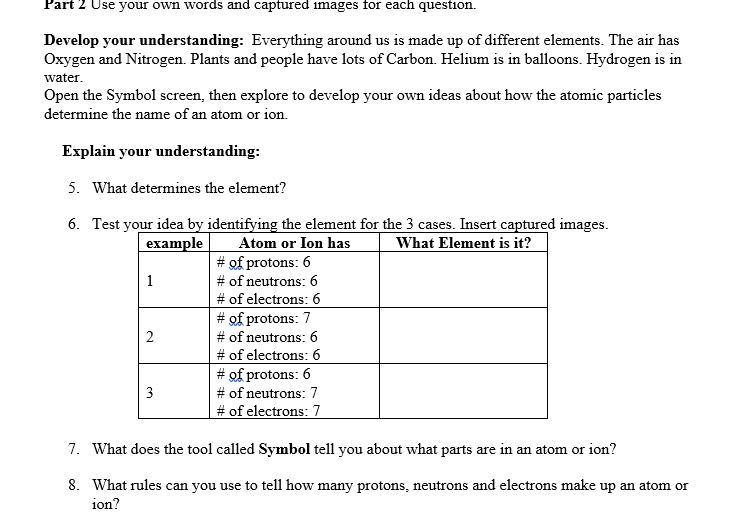
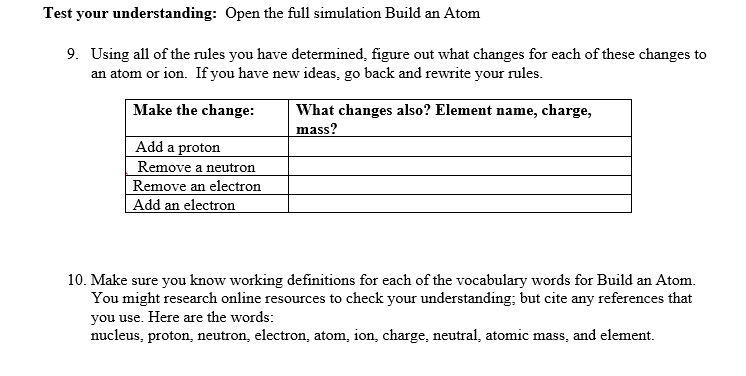
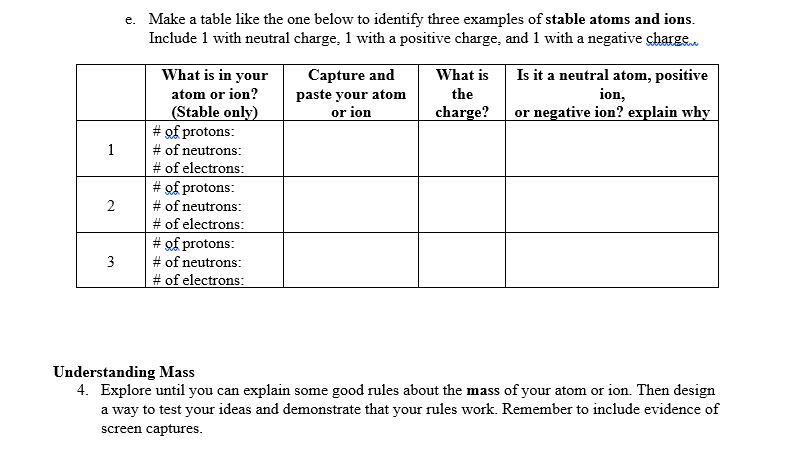
This lab uses the Build an W from MI Interactive-e Simulations at University of lColorado Boulder, under the CC-BY 4i} license- Simulation Link: hwcoloradoedufsimsfhtll!'ldanatom'iatestfbuildanatom en.html 0 Learning Goals: Students will be able to 1. Make atom models that show stable atoms or ions. 2. Use given information about subatomic particles to i Identif)r an element and its position on the periodic table II Draw models m I Determine il'the model is for a neutral atom or an ion. 3. Predict how addition or subtraction of a proton, neutron, or electron will change the element, the charge: and the mass oftheir atom or ion. 4. Describe all vocabulary words needed to meet the goals. Part 1 Directions: Use your own words and captured images for each question. Develop your understanding: Open the Atom screen, then explore to develop your own ideas about the atomic particles that make up atoms and ions. Explain your understanding: 1. 1What parts go inthe center ofthe atom? 'What is the center called? 2. Explore until you discover a good rule for making the center of the atom stable. 2. Explore until you discover a good rule for making the center of the atom stable. at. What seems to make the center of the atom unstable? b. Fill in the table to identify,r three examples at least 1 stable and at least 1 unstable that shows your rules for stabilityr work to \"build an atom" of your own. _ Capture and Is it stable or 'What Element is nucleus? :aste nur atom unstable? it? Develop your understanding: Explore to develop your own ideas about what makes an atom and how ions are different from atoms. Explain your understanding: 3. What are some good rules about the charge ofvo'ur atom or ion? a. What is a rule for making a neutral atom which has no charge? b. What is a rule for making a positive ion which has positive charge? c. What is a rule for making a negative ion which has negative charge? d. How did you use the tools in the sim that helped you decide if the atom had a positive, negative, or 0 charge? fart I Use your own words and captured images tor each question. ew-lop your widen-standing: Everything around us is made up of different elements- The air has Oxygen and Nitrogen. Plants and people have lots of Carbon. Helium is in balloons- Hydrogen is in water. lDpen the Symbol screen, then explore to develop your own ideas about houtr the atomic particles determine the name of an atom or ion. Explain your understanding: 5. What determines the element? # of neutrons: # of electrons: 6 7'. 'What does the tool called Symbol be]! you about what parts are in an atom or ion? 8. What rules can you use to tell how many protons, neutrons and electrons make up an atom or ion? Test your understanding: Open the full simulation Build an Atom 9. Using all of the rules you have lietermineti, gure out what changes for each of these changes to an atom or ion. If you have new ideas, go back and reunite your rules. Make the change: \"int changes also? Element name, charge, mass? . Remove neutron 1G. Make sure you know working denitions for each of the VDChlllIy words for Build an Atom. You might research oniiue resources to check your understanding; but cite any references that you use. Here are the words: nucleus= proton, neutron, electron, atom, ion, charge, neutral= atonlic mass, and element. e. Make a table like the one below to ideicittifjl.r three examples of stable atoms and ions. Inelude l with neutral charge, 1 with a positive charge, and 1 with a negative Me\" What: is in your Capture and What is Is it a neutral atom, positive atom or inn? ion, {Stable only} . or negative ion? explain why # prrotons: # of nemrons: # of electrons: Understanding Mass 4. Explore until you can explain some good rules about the mass of your atom or ion. Then design a way to test your ideas and demonstrate that your rules work. Remember to include evidence of screen captures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


