Question: this problem comes from a thermodynamics class that is currently covering combustion and chemical reactions Please answer the following questions as well. I will leave
this problem comes from a thermodynamics class that is currently covering combustion and chemical reactions
Please answer the following questions as well. I will leave a good review!
b) Calculate the Lower Heating Value for the coal above
c) What is the air fuel ratio in lbm air/ lbm fuel?
d) Calculate the dew point of the products of combustion (ignore the ash in the products of combustion).
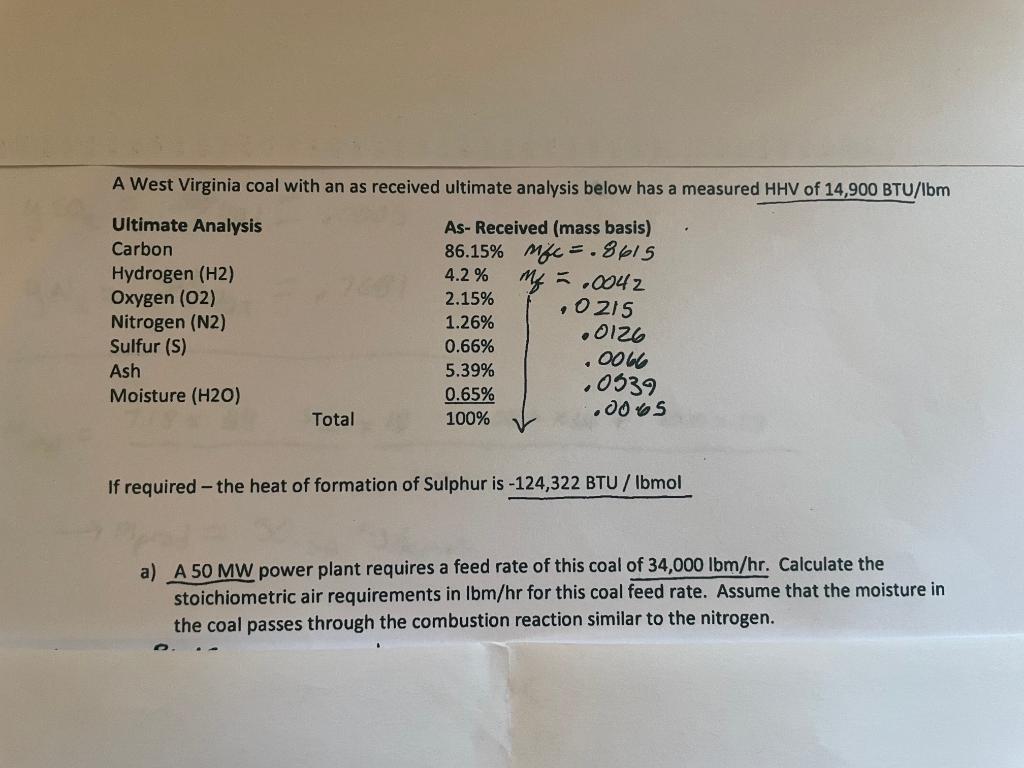
A West Virginia coal with an as received ultimate analysis below has a measured HHV of 14,900 BTU/\bm Ultimate Analysis Carbon Hydrogen (H2) Oxygen (02) Nitrogen (N2) Sulfur (S) Ash Moisture (H20) As-Received (mass basis) 86.15% Mfc = .8615 4.2 % me = .0042 2.15% 0215 1.26% .0126 0.66% 5.39% .0066 .0539 0.65% 0065 100% Total If required - the heat of formation of Sulphur is -124,322 BTU/ Ibmol a) A 50 MW power plant requires a feed rate of this coal of 34,000 lbm/hr. Calculate the stoichiometric air requirements in Ibm/hr for this coal feed rate. Assume that the moisture in the coal passes through the combustion reaction similar to the nitrogen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


