Question: This question has 2 pictures attached. Devide your answer to 8 section as in the question and add short and understandable explanations. Question 3 -
This question has 2 pictures attached. Devide your answer to 8 section as in the question and add short and understandable explanations.
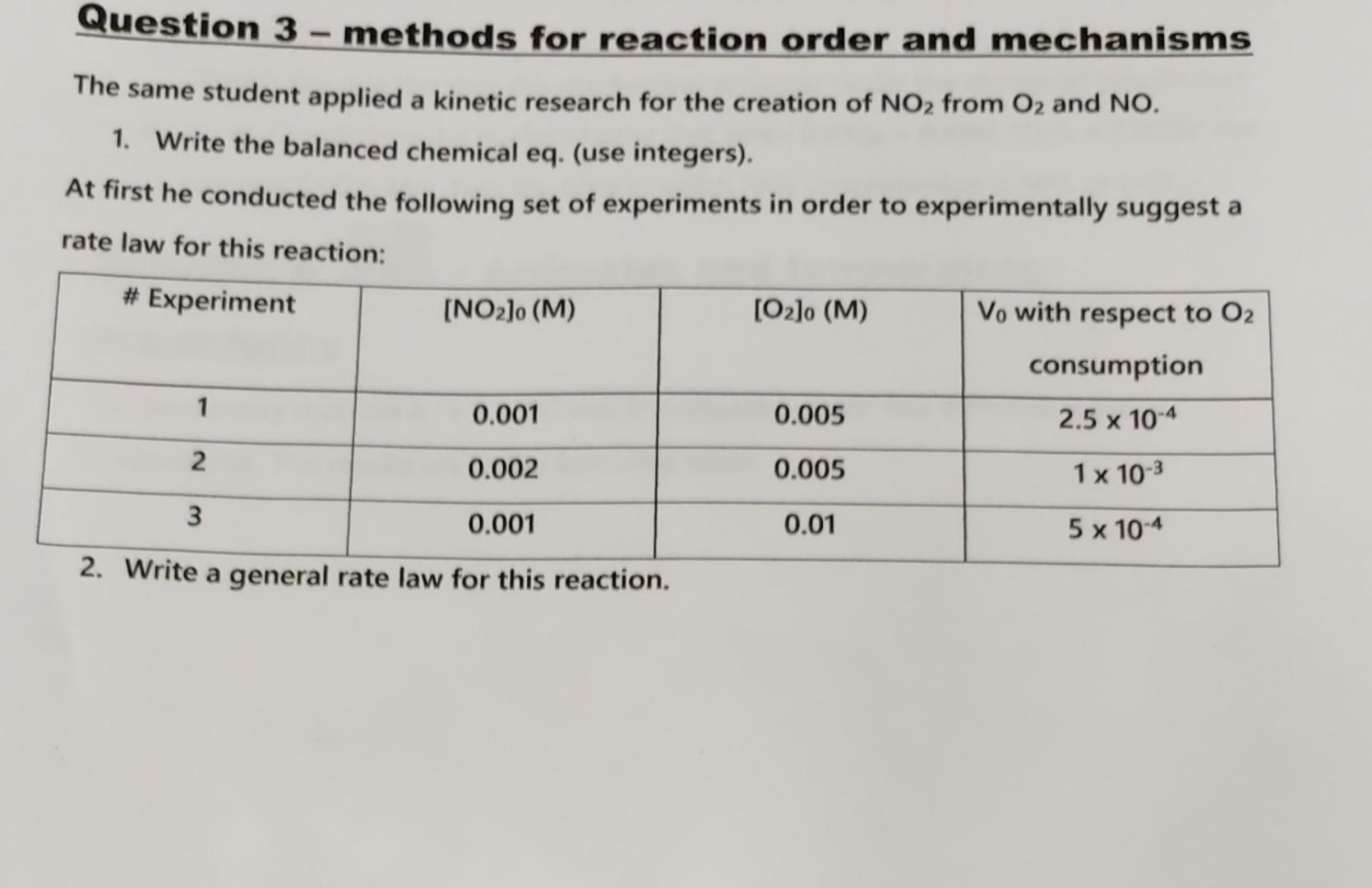

Question 3 - methods for reaction order and mechanisms The same student applied a kinetic research for the creation of NO2 from O2 and NO. 1. Write the balanced chemical eq. (use integers). At first he conducted the following set of experiments in order to experimentally suggest a rate law for this reaction: c. vvrite a general rate law for this reaction. 3. Use the data in the table to calculate the order of the reaction with respect to each of the reactants. What is the total order of this reaction? 4. Calculate the reaction rate constant in these experiment conditions. n order to explain the rate law, the student suggested the following mechanism: NO+NOk1N2O2N2O2k22NON2O2+O2k12NO2 (The reactions are I, II and III respectively). 5. According to this mechanism, find an expression for the rate of the reaction, i.e. for d[NO2]/dt using [NO2], [O2] and k1,k2 and k3 solely. (Use S.S assumption for intermediates). 6. Show that the suggested mechanism explains the experimental rate law only when reaction III is way faster than reaction II (i.e. k3>>k2 ). What is the rate constant of the reaction in terms of k1,k2 and k3 ? 7. In certain conditions (let's call it set 2), different than those in which the student here worked, k2>>k3 What is the rate law that this mechanism supports under the discussed conditions? 8. In set 2 conditions the student found that when [NO2]0=0.01M,[O2]0=0.005M the rate was 0.0015M/s. Find the time in which NO2 concentration is 50% of [NO2]0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


