Question: This question is about treating nerve agent poisoning Nerve agents bind to and inhibit an enzyme called acetylcholinesterase ( AChE ) . The inhibited AChE
This question is about treating nerve agent poisoning
Nerve agents bind to and inhibit an enzyme called
acetylcholinesterase AChE The inhibited AChE can no longer
hydrolyse a key neurotransmitter, which leads to paralysis and
ultimately death.
One of the main treatments for nerve agent poisoning is a group
of chemicals containing the oxime functional group NOH
These reactivate the inhibited AChE by removing the bound nerve
agent. An important reactivator is the salt pyridine aldoxime
methyl chloride PAM
ai Calculate the molar mass of PAM.
ii An adult poisoned by a nerve agent requires an hourly dose of mmol of PAM
for every kilogram of body mass. Calculate the mass of PAM required for an
person over a hour treatment period.
Nerve agents work very quickly. Kinetic studies of oximes treatments are an important area of
study. The rate of reactivation of inhibited AChE by PAM is shown below.
bi Give the approximate order of reaction with respect to PAM at concentrations of
PAM below
ii Give the approximate order of reaction with respect to PAM at concentrations of
PAM above
The following twostep mechanism was proposed to explain these results:
AChEI PAM AChEPAM AChE PAM
where AChEI is the inhibited AChE, AChEIPAM is a complex of the inhibited AChE and
PAM, and IPAM is PAM with the nerve agent attached.
c is the equilibrium constant for the the first step. Write an expression for
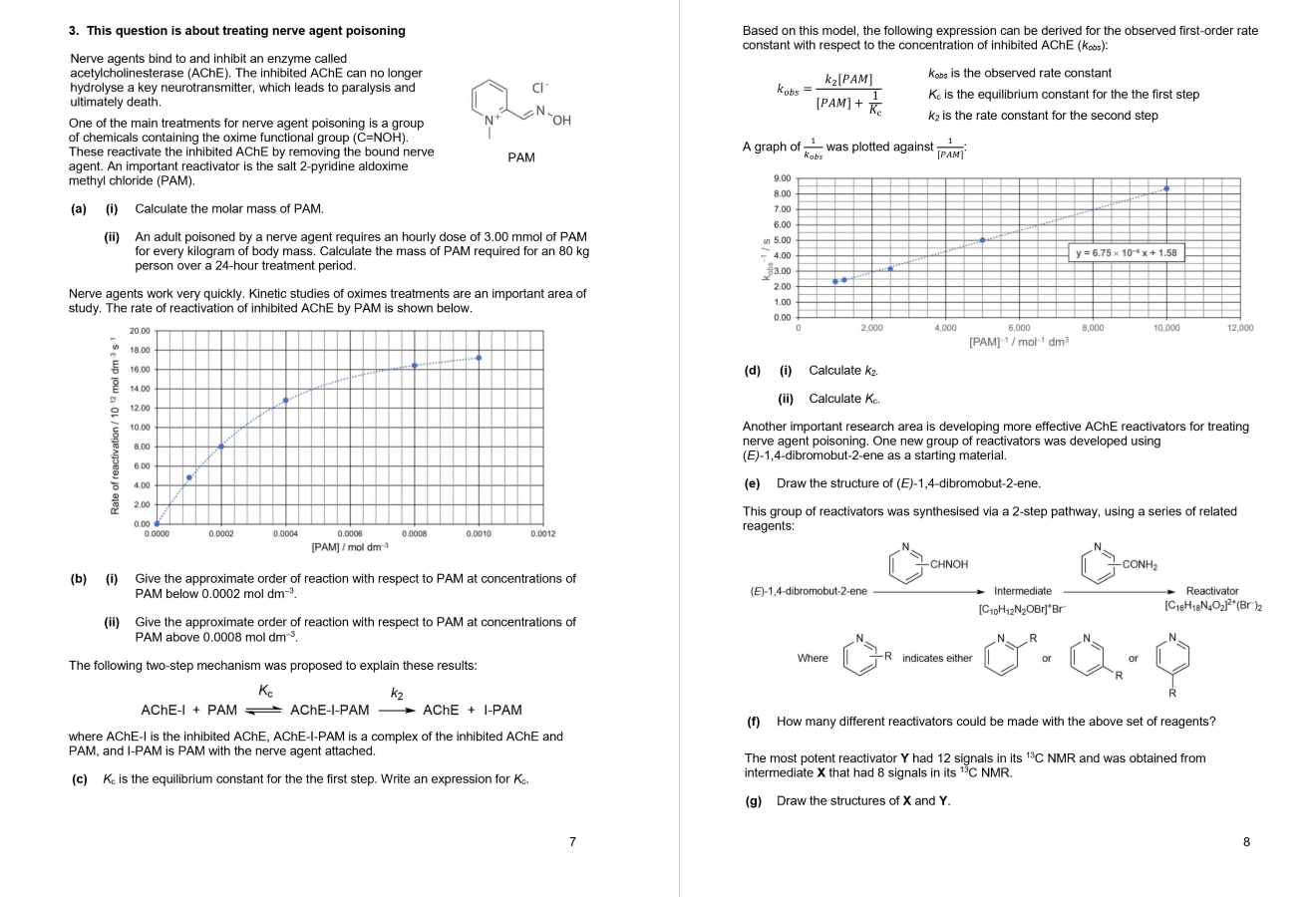
Based on this model, the following expression can be derived for the observed firstorder rate
constant with respect to the concentration of inhibited AChE : :
is the observed rate constant
is the equilibrium constant for the the first step
is the rate constant for the second step
A graph of was plotted against :
di Calculate
ii Calculate
Another important research area is developing more effective AChE reactivators for treating
nerve agent poisoning. One new group of reactivators was developed using
Edibromobutene as a starting material.
e Draw the structure of dibromobutene.
This group of reactivators was synthesised via a step pathway, using a series of related
reagents:
E
f How many different reactivators could be made with the above set of reagents?
The most potent reactivator had signals in its NMR and was obtained from
intermediate that had signals in its NMR
g Draw the structures of and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


