Question: This question is asked earlier In first part show the plot and calculate the values of Cao, CBO, CCO after 10 seconds In second part
This question is asked earlier In first part show the plot and calculate the values of Cao, CBO, CCO after 10 seconds In second part find maximum concentration of B and show all the plots that were asked
please give complete answer, otherwise I will dislike.
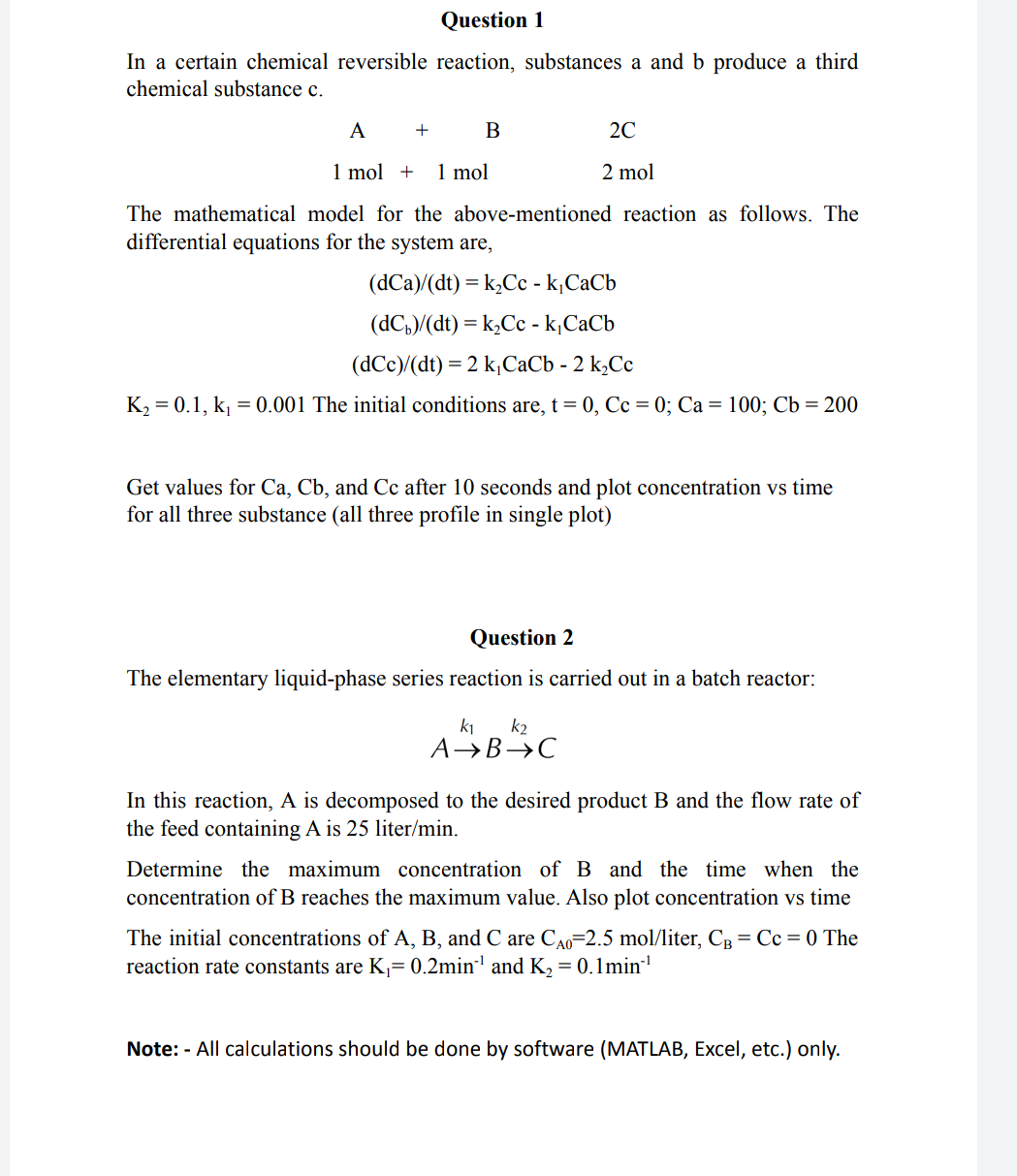
In a certain chemical reversible reaction, substances a and b produce a third chemical substance c. The mathematical model for the above-mentioned reaction as follows. The differential equations for the system are, (dCa)/(dt)=k2Cck1CaCb(dCb)/(dt)=k2Cck1CaCb(dCc)/(dt)=2k1CaCb2k2Cc K2=0.1,k1=0.001 The initial conditions are, t=0,Cc=0;Ca=100;Cb=200 Get values for Ca,Cb, and Cc after 10 seconds and plot concentration vs time for all three substance (all three profile in single plot) Question 2 The elementary liquid-phase series reaction is carried out in a batch reactor: Ak1Bk2C In this reaction, A is decomposed to the desired product B and the flow rate of the feed containing A is 25 liter/min. Determine the maximum concentration of B and the time when the concentration of B reaches the maximum value. Also plot concentration vs time The initial concentrations of A,B, and C are CA0=2.5mol/ liter, CB=Cc=0 The reaction rate constants are K1=0.2min1 and K2=0.1min1 Note: - All calculations should be done by software (MATLAB, Excel, etc.) only
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


