Question: this question need to be done perfectly.answer is given below so match your answer before submission thanks 3. A certain natural gas has the following
 this question need to be done perfectly.answer is given below so match your answer before submission thanks
this question need to be done perfectly.answer is given below so match your answer before submission thanks
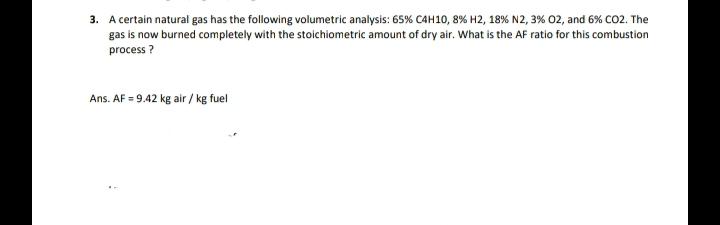
3. A certain natural gas has the following volumetric analysis: 65% C4H10,8% H2, 18% N2, 3% 02, and 6% CO2. The gas is now burned completely with the stoichiometric amount of dry air. What is the AF ratio for this combustion process? Ans. AF = 9.42 kg air / kg fuel
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


