Question: this report is example. write looks like this report? (As experimental, aim of experiment, conclusion.) 1 Student/Class/ClassDetail?classid=a6924d3df9c3ab2b&courseld=5941 8 Demonstration Experiments 1-Chemical decomposition of hydrogen peroxide
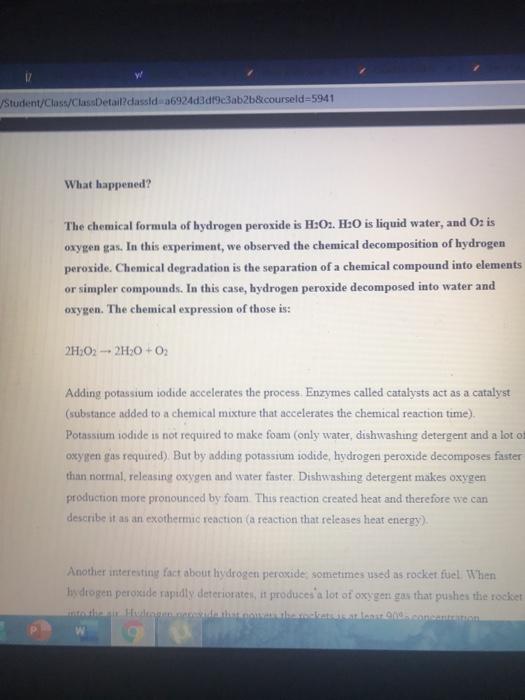





1 Student/Class/ClassDetail?classid=a6924d3df9c3ab2b&courseld=5941 8 Demonstration Experiments 1-Chemical decomposition of hydrogen peroxide by catalysis Hydrogen peroxide 50% of the liquid detergent as a result of the reaction with the mixing of the oxygen gas is released with foam Under normal conditions, this reaction takes several hours to complete. However, when the Potassium Iodide catalyst is added to the reaction, the process takes place in a few seconds As a result of the reaction, a very colorful and visual experiment with plenty of activity occurs. We recommend you to perform this experiment in an open area What do we need? Protective Glasses Graduated cylinder 500 ml Erlenmeyer flask 200 ml Examination Gloves Plastic Spatulas Liquid dishwashing detergent Hydrogen peroxide 50% Potassium iodide Food color . . /Student/Class/ClassDetaildassid 36924d3d9c3ab2b&courseld=5941 What happened? The chemical formula of hydrogen peroxide is H:02. H:O is liquid water, and O is oxygen gas. In this experiment, we observed the chemical decomposition of hydrogen peroxide. Chemical degradation is the separation of a chemical compound into elements or simpler compounds. In this case, hydrogen peroxide decomposed into water and oxygen. The chemical expression of those is: 2H202 - 2H30+02 Adding potassium iodide accelerates the process. Enzymes called catalysts act as a catalyst (substance added to a chemical mixture that accelerates the chemical reaction time) Potassium iodide is not required to make form (only water, dishwashing detergent and a lot of oxygen gas required) But by adding potassium iodide, hydrogen peroxide decomposes faster than normal, releasing oxygen and water faster Dishwashing detergent makes oxygen production more pronounced by foam This reaction created heat and therefore we can describe it as an exothermic reaction (a reaction that releases heat energy). Another interesting fact about hydrogen peroxide sometimes used as rocket fuel When hydrogen peroxide rapidly deteriorate, it produces a lot of oxygen gas that pushes the rocket lite od Hetticasinot.comcanton 1S/Student/Class/ClassDetail?classid=a6924d3df9c3ab2b&courseld=5941 TKOZ21.020501 TEKM HOROJEN PEROKSET Herode yan 23LL oturumlarnza ogrenci -d8b9-4dd0-a7de-40719227a0a4.mp4 40 Care RE Posch 0 kurumlarnzarOgrence big ekrannda yer alan tuan bersien W b ferfresh Beyaz Sirke White Vinegar 1000, Aamur Kabartma Tozu Yeni tariflerle 0.16 0.22 1 Student/Class/ClassDetail?classid=a6924d3df9c3ab2b&courseld=5941 8 Demonstration Experiments 1-Chemical decomposition of hydrogen peroxide by catalysis Hydrogen peroxide 50% of the liquid detergent as a result of the reaction with the mixing of the oxygen gas is released with foam Under normal conditions, this reaction takes several hours to complete. However, when the Potassium Iodide catalyst is added to the reaction, the process takes place in a few seconds As a result of the reaction, a very colorful and visual experiment with plenty of activity occurs. We recommend you to perform this experiment in an open area What do we need? Protective Glasses Graduated cylinder 500 ml Erlenmeyer flask 200 ml Examination Gloves Plastic Spatulas Liquid dishwashing detergent Hydrogen peroxide 50% Potassium iodide Food color . . /Student/Class/ClassDetaildassid 36924d3d9c3ab2b&courseld=5941 What happened? The chemical formula of hydrogen peroxide is H:02. H:O is liquid water, and O is oxygen gas. In this experiment, we observed the chemical decomposition of hydrogen peroxide. Chemical degradation is the separation of a chemical compound into elements or simpler compounds. In this case, hydrogen peroxide decomposed into water and oxygen. The chemical expression of those is: 2H202 - 2H30+02 Adding potassium iodide accelerates the process. Enzymes called catalysts act as a catalyst (substance added to a chemical mixture that accelerates the chemical reaction time) Potassium iodide is not required to make form (only water, dishwashing detergent and a lot of oxygen gas required) But by adding potassium iodide, hydrogen peroxide decomposes faster than normal, releasing oxygen and water faster Dishwashing detergent makes oxygen production more pronounced by foam This reaction created heat and therefore we can describe it as an exothermic reaction (a reaction that releases heat energy). Another interesting fact about hydrogen peroxide sometimes used as rocket fuel When hydrogen peroxide rapidly deteriorate, it produces a lot of oxygen gas that pushes the rocket lite od Hetticasinot.comcanton 1S/Student/Class/ClassDetail?classid=a6924d3df9c3ab2b&courseld=5941 TKOZ21.020501 TEKM HOROJEN PEROKSET Herode yan 23LL oturumlarnza ogrenci -d8b9-4dd0-a7de-40719227a0a4.mp4 40 Care RE Posch 0 kurumlarnzarOgrence big ekrannda yer alan tuan bersien W b ferfresh Beyaz Sirke White Vinegar 1000, Aamur Kabartma Tozu Yeni tariflerle 0.16 0.22
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


