Question: This was a problem on an old midterm exam. I suggest you do it by hand and get all properties from your water tables. Water
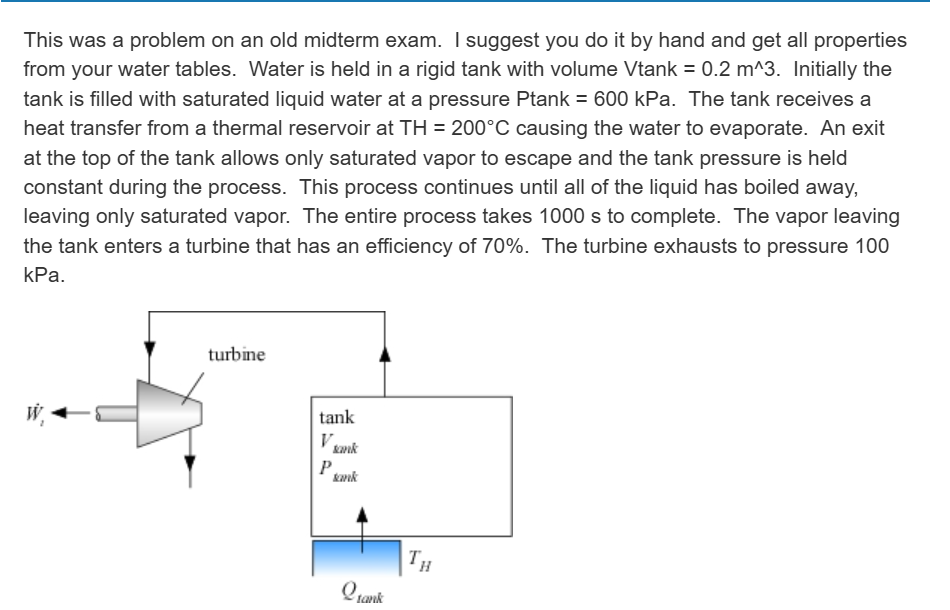
This was a problem on an old midterm exam. I suggest you do it by hand and get all properties
from your water tables. Water is held in a rigid tank with volume Vtank m Initially the
tank is filled with saturated liquid water at a pressure Ptank kPa. The tank receives a
heat transfer from a thermal reservoir at TH@C causing the water to evaporate. An exit
at the top of the tank allows only saturated vapor to escape and the tank pressure is held
constant during the process. This process continues until all of the liquid has boiled away,
leaving only saturated vapor. The entire process takes s to complete. The vapor leaving
the tank enters a turbine that has an efficiency of The turbine exhausts to pressure
kPa.
a What is the temperature of the water in the tank C
b What is the mass of water that leaves the tank kg
c What is the total heat transfer to the tank kJ
d What is the mass flow rate leaving the tank kgs
e What is the rate of power produced by the turbine kW
f What is the total amount of entropy generated by the process kJKBe careful of the system you are using you do NOT have to account for any entropy generation that occurs downstream of the turbine exit.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


