Question: this was the only data provided Incorrect. What is known as conjugated T-system? Identify the stability of the terminal and the internal furans. During a
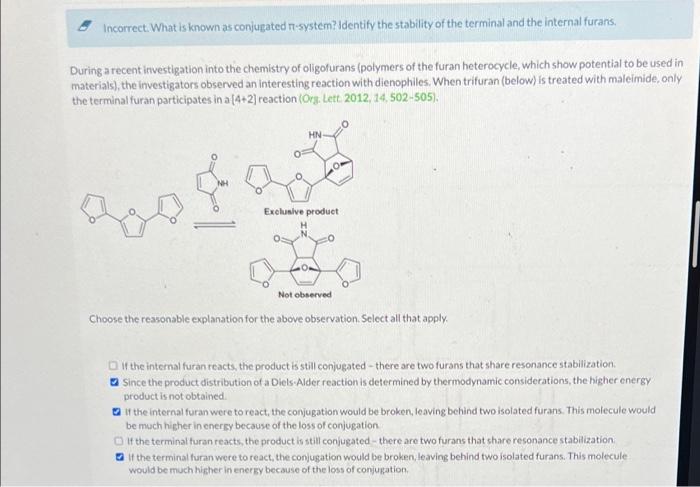
Incorrect. What is known as conjugated T-system? Identify the stability of the terminal and the internal furans. During a recent investigation into the chemistry of oligofurans (polymers of the furan heterocycle, which show potential to be used in materials), the investigators observed an interesting reaction with dienophiles. When trituran (below) is treated with maleimide, only the terminal furan participates in a [4+2) reaction (Org. Lett. 2012, 14, 502-505). HN . Exclusive product -0 Not observed Choose the reasonable explanation for the above observation Select all that apply if the internal furan reacts, the product is still conjugated there are two furans that share resonance stabilization Since the product distribution of a Diels-Alder reaction is determined by thermodynamic considerations, the higher energy product is not obtained at the internal furan were to react, the conjugation would be broken, leaving behind two isolated furans. This molecule would be much higher in energy because of the loss of conjugation If the terminal turan reacts the product is still conjugated there are two furans that share resonance stabilization of the terminaluran were to react, the conjugation would be broken, leaving behind two isolated furans. This molecule would be much higher in energy because of the loss of conjugation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


