Question: Titanium (Phi ) = ( 6.94times 10^(-19)(J)) and silicon (Phi ) = ( 7.77times 10^(-19)(J)) surfaces are irradiated with UV radiation with a wavelength of
Titanium
(\\\\Phi )
=(
6.94\\\\times 10^(-19)(J))and silicon
(\\\\Phi )
=(
7.77\\\\times 10^(-19)(J))surfaces are irradiated with UV radiation with a wavelength of
229nm.\ 1st attempt\ Part 1 (1 point)\ See Periodic Table\ See Hint\ What is the wavelength of the electrons emitted by the titanium surface?\
nm\ Part 2 (1 point)\ See Hint\ Which metal emits electrons with the longer wavelength?\ Choose one:\ Si\ Ti\ Both emit the same wavelength.
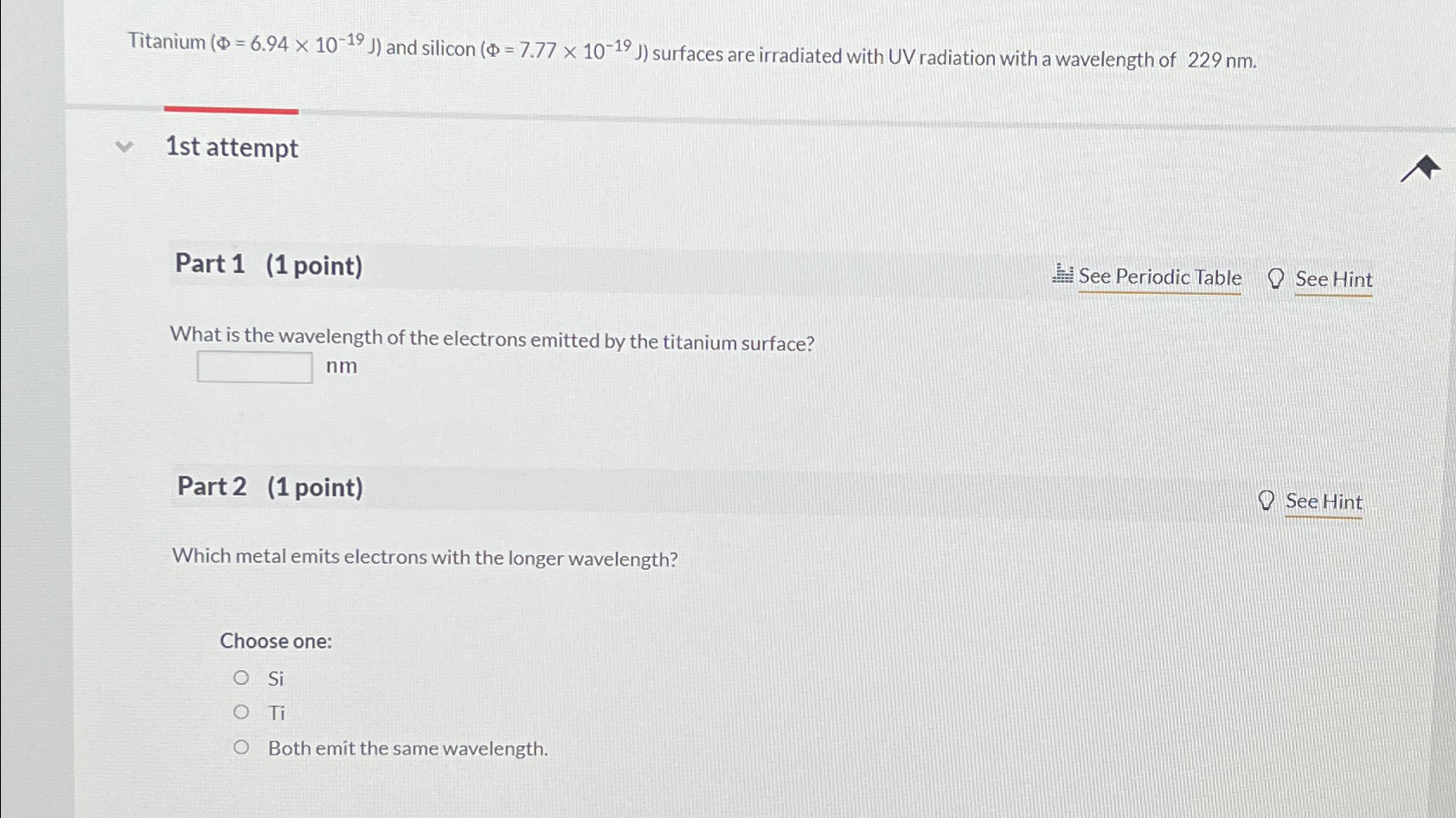
Titanium ( =6.941019J) and silicon ( =7.771019J) surfaces are irradiated with UV radiation with a wavelength of 229nm. 1st attempt Part 1 (1 point) What is the wavelength of the electrons emitted by the titanium surface? nm Part 2 (1 point) Which metal emits electrons with the longer wavelength? Choose one: Si Ti Both emit the same wavelength
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


