Question: Title =q2a6 The reaction A+BC is found to be first order with respect to A and zero order with respect to B. When the concentration
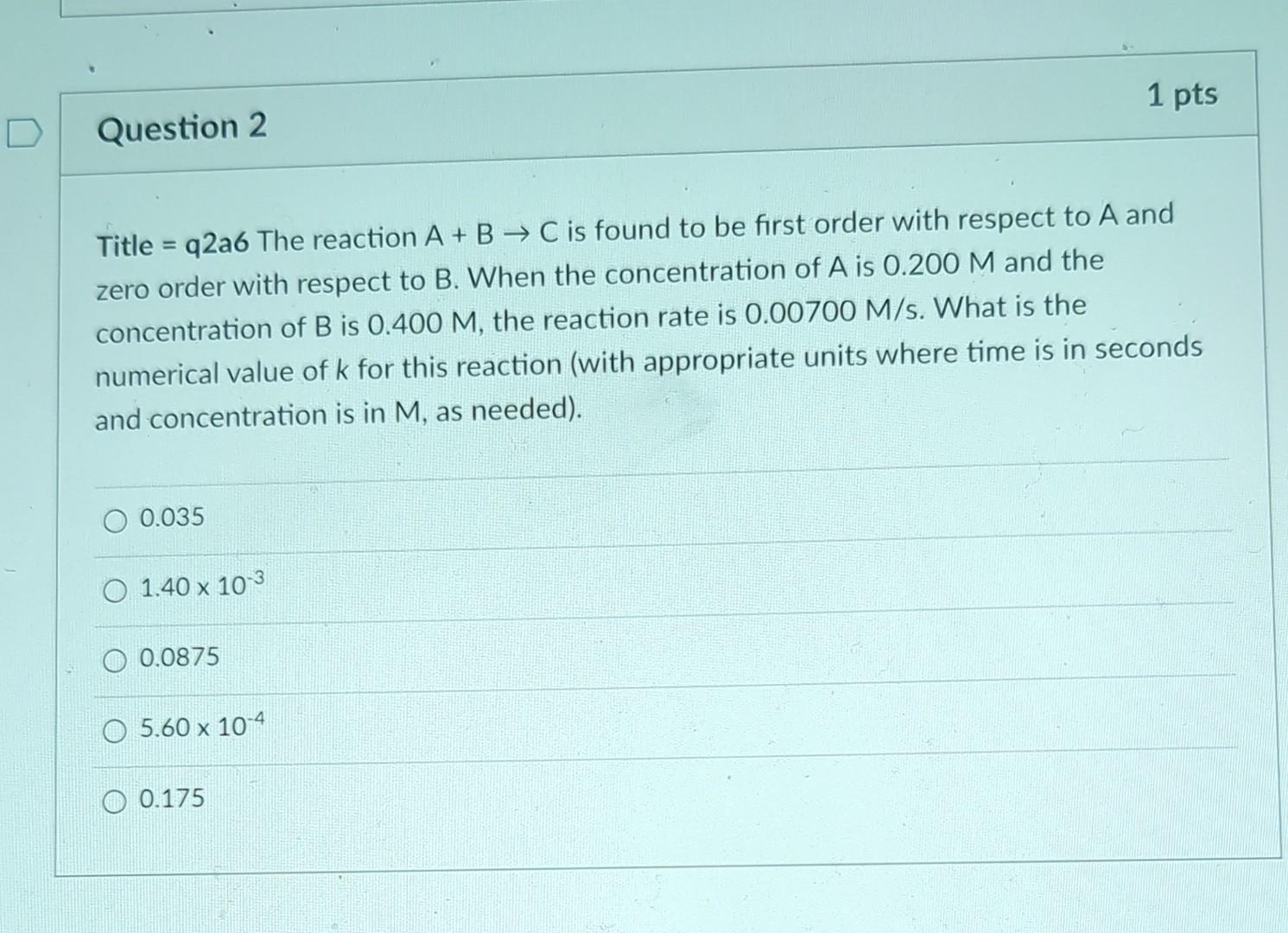
Title =q2a6 The reaction A+BC is found to be first order with respect to A and zero order with respect to B. When the concentration of A is 0.200M and the concentration of B is 0.400M, the reaction rate is 0.00700M/s. What is the numerical value of k for this reaction (with appropriate units where time is in seconds and concentration is in M, as needed). 0.0351.401030.08755.601040.175
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


