Question: To how many significant figures should each answer be rounded? (6.626 x 10-24J - s) (2.9979 x 108 m/s) equation A: = 4.404453525499 x 10-19](unrounded)
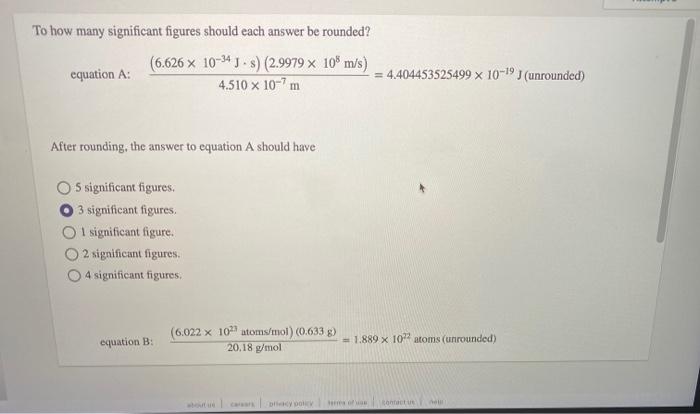

To how many significant figures should each answer be rounded? (6.626 x 10-24J - s) (2.9979 x 108 m/s) equation A: = 4.404453525499 x 10-19](unrounded) 4.510 x 10-7m After rounding, the answer to equation A should have S significant figures. 3 significant figures 1 significant figure. 2 significant figures 4 significant figures equation B: (6.022 X 10atoms/mol) (0.6338) = 1.889 X 107 atoms (unrounded) 20.18 g/mol Contacte equation B: (6.022 X 102 atoms/mol) (0.633 g) 20.18 g/mol = 1.889 x 1022 atoms (unrounded) After rounding the answer to equation B should have 3 significant figures. 2 significant figures. 4 significant figures. 5 significant figures 1 significant figure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


