Question: To make a 0.500 M solution, one could take 0.500 moles of solute and add Qa 1.00 L of solvent Ob. 1.00 kg of solvent
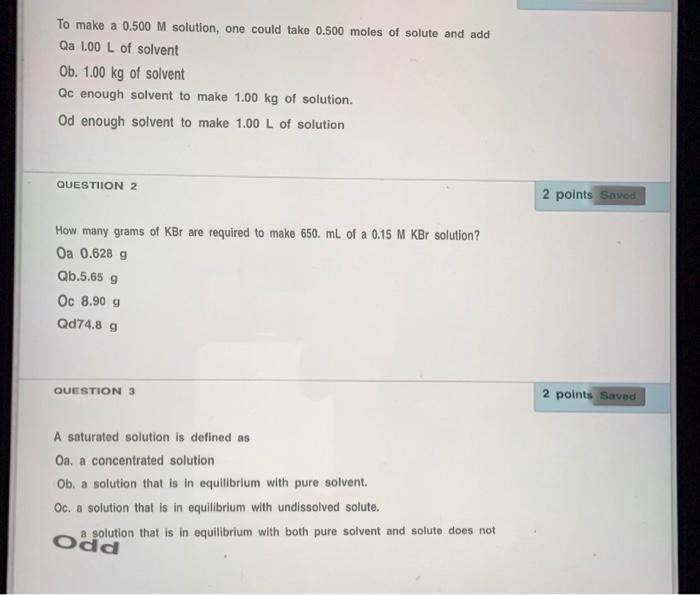
To make a 0.500 M solution, one could take 0.500 moles of solute and add Qa 1.00 L of solvent Ob. 1.00 kg of solvent Qc enough solvent to make 1.00 kg of solution. Od enough solvent to make 1.00 L of solution QUESTION 2 2 points Saved How many grams of KBr are required to make 650. mL of a 0.15 M KBr solution? Oa 0.628 g Qb.5.65 g Oc 8.90 g Qd74.8 g QUESTION 3 2 points Saved A saturated solution is defined as Oa a concentrated solution Ob. a solution that is in equilibrium with pure solvent. Oc. a solution that is in equilibrium with undissolved solute. a solution that is in equilibrium with both pure solvent and solute does not odd
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


