Question: To make beer, a solution called wort is fermented to produce ethanol, C2H5OH. C6H1206 + 2 CO2 + 2 C H OH What is the
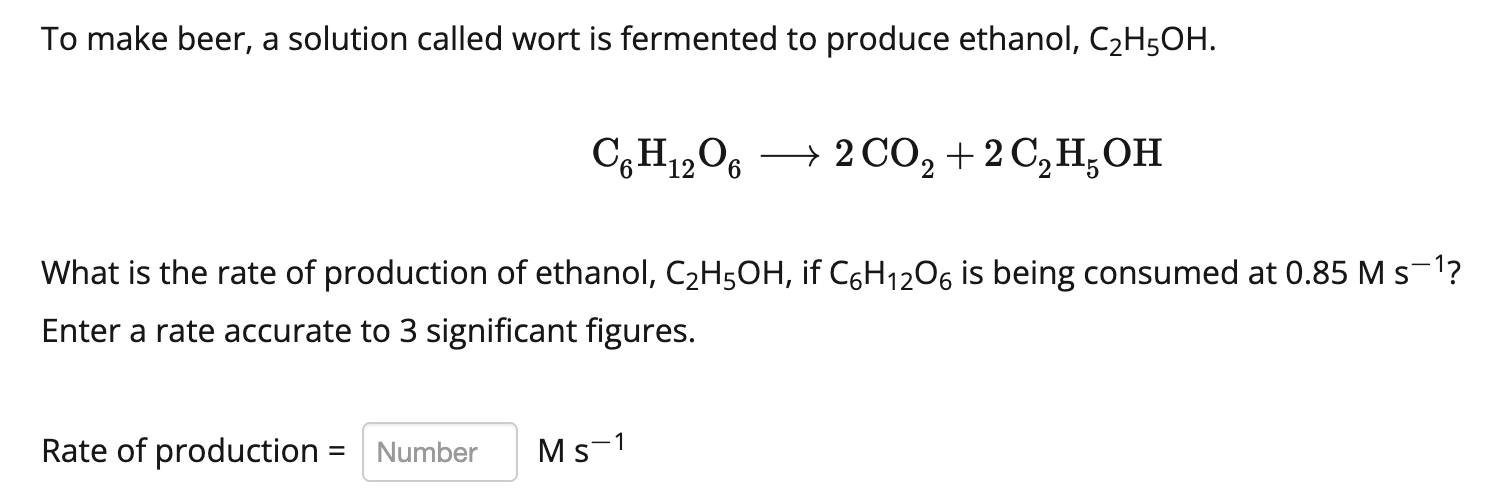
To make beer, a solution called wort is fermented to produce ethanol, C2H5OH. C6H1206 + 2 CO2 + 2 C H OH What is the rate of production of ethanol, C2H5OH, if C6H1206 is being consumed at 0.85 M s-1? Enter a rate accurate to 3 significant figures. Rate of production = Number Ms-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


