Question: Total Volume Recovered: SIMPLE: FRACTIONAL: Draw a single graph on which you will plot the number of drops on the X-axis and Temperature on the
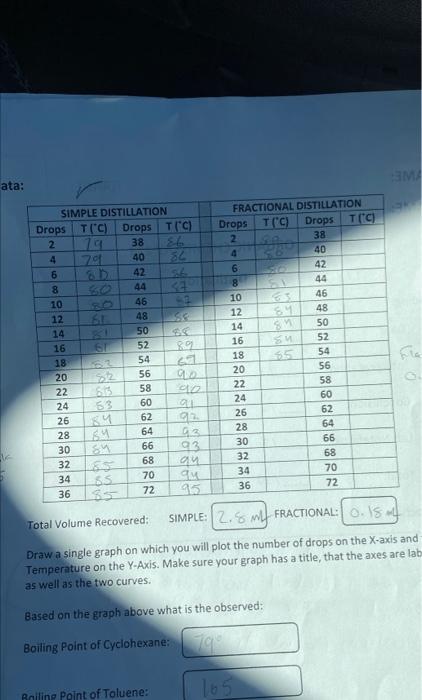
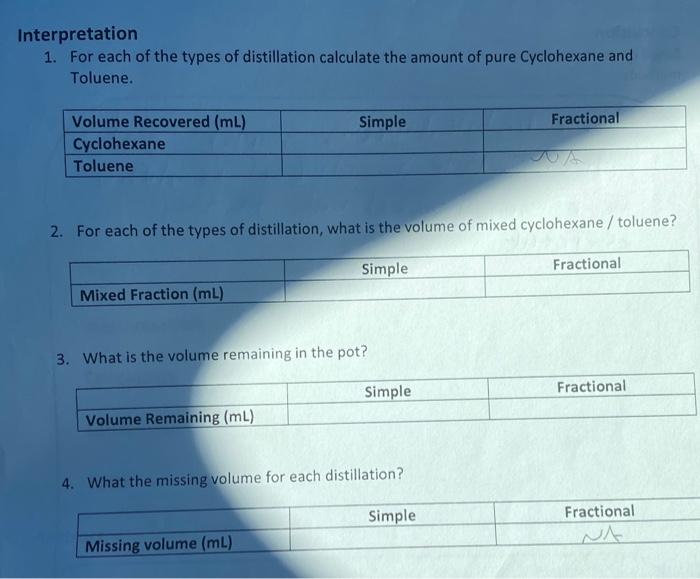
Total Volume Recovered: SIMPLE: FRACTIONAL: Draw a single graph on which you will plot the number of drops on the X-axis and Temperature on the Y-Axis. Make sure your graph has a title, that the axes are lab as well as the two curves. Based on the graph above what is the observed: Boiling Point of Cyclohexane: Railing Point of Toluene: nterpretation 1. For each of the types of distillation calculate the amount of pure Cyclohexane and Toluene. 2. For each of the types of distillation, what is the volume of mixed cyclohexane / toluene? 3. What is the volume remaining in the pot? 4. What the missing volume for each distillation? Total Volume Recovered: SIMPLE: FRACTIONAL: Draw a single graph on which you will plot the number of drops on the X-axis and Temperature on the Y-Axis. Make sure your graph has a title, that the axes are lab as well as the two curves. Based on the graph above what is the observed: Boiling Point of Cyclohexane: Railing Point of Toluene: nterpretation 1. For each of the types of distillation calculate the amount of pure Cyclohexane and Toluene. 2. For each of the types of distillation, what is the volume of mixed cyclohexane / toluene? 3. What is the volume remaining in the pot? 4. What the missing volume for each distillation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


