Question: Using the given data, calculate the rate constant of this reaction. A + B k= Number 0.580 C + D Units M-2S-1 Trial [A]
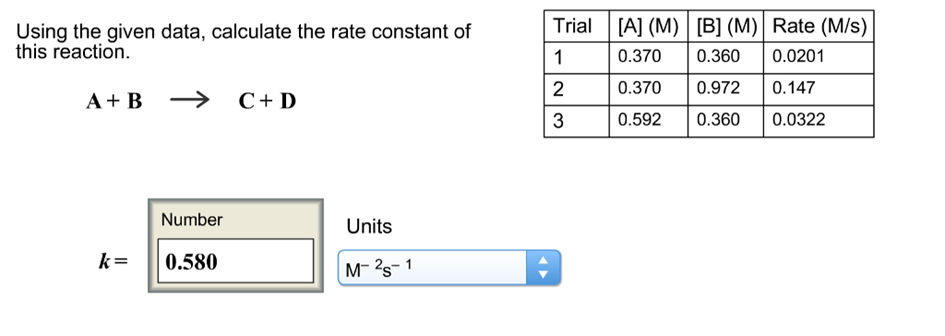
Using the given data, calculate the rate constant of this reaction. A + B k= Number 0.580 C + D Units M-2S-1 Trial [A] (M) [B] (M) Rate (M/s) 1 0.370 0.360 0.0201 2 0.370 0.972 0.147 3 0.592 0.360 0.0322
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
Solution By the table Trial A M B M Rate Ms 1 0370 0360 00201 2 ... View full answer

Get step-by-step solutions from verified subject matter experts


