Question: Trial Using the data in the table, calculate the rate constant of this reaction. [B] (M) 0.320 Rate (M/s) 0.0101 1 (A) (M) 0.360 0.360
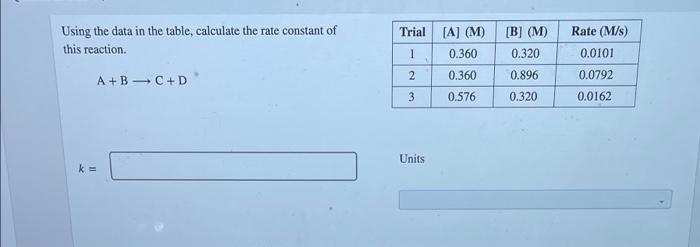
![of this reaction. [B] (M) 0.320 Rate (M/s) 0.0101 1 (A) (M)](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f857d4aabe6_44466f857d45581c.jpg)
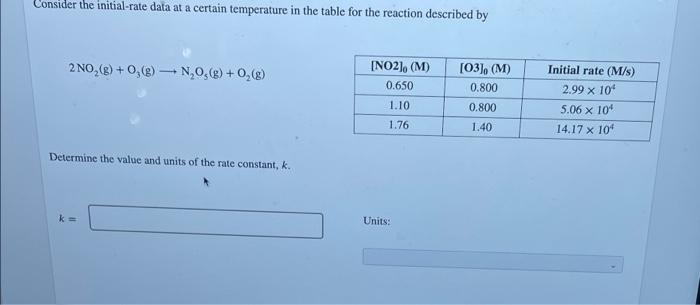
Trial Using the data in the table, calculate the rate constant of this reaction. [B] (M) 0.320 Rate (M/s) 0.0101 1 (A) (M) 0.360 0.360 0.576 2 0.896 0.0792 A+B C+D 3 0.320 0.0162 Units k = Using the data in the table, determine the rate constant of the reaction and select the appropriate units. A +2B C+D Trial [A] (M) [B] (M) Rate (M/s) 1 0.260 0.270 0.0154 2 0.260 0.540 0.0154 3 0.520 0.270 0.0616 0.844 Units k= Incorrect M-'s-1 Consider the initial-rate data at a certain temperature in the table for the reaction described by 2 NO,() + 0,0) + NO(g) + O2(%) - INO2). (M) 0.650 [03), (M) 0.800 0.800 1.40 1.10 Initial rate (M/s) 2.99 x 10 5.06 x 10' 14.17 x 10 1.76 Determine the value and units of the rate constant, k. ks Units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


