Question: Tried and couldn't figure it out A flask is charged with 1.300 atm of N2O4(g) and 1.15atmNO2(g) at 25C. The equilibrium reaction is given in
Tried and couldn't figure it out 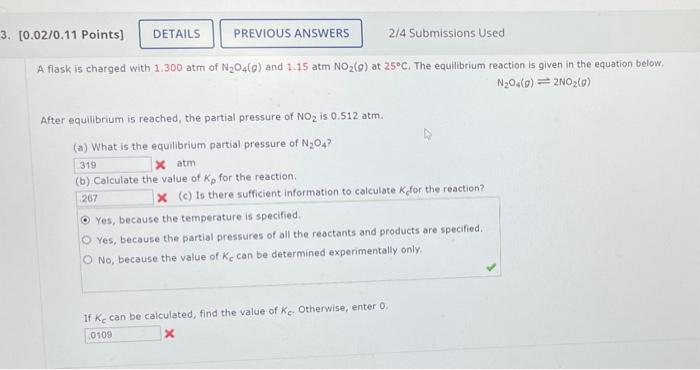

A flask is charged with 1.300 atm of N2O4(g) and 1.15atmNO2(g) at 25C. The equilibrium reaction is given in the equation below. N2O4(g)2NO2(g) After equilibrium is reached, the partial pressure of NO2 is 0.512atm. (a) What is the equilibrium partial pressure of N2O4 ? (b) Calculate the value of Kp for the reaction. x (c) Is there sufficient information to calculate K for the reaction? Yes, because the temperature is specified. Yes, because the partial pressures of all the reactants and products are specified. No, because the value of Kc can be determined experimentally only. If Kc can be calculated, find the value of Kc. Otherwise, enter 0
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


