Question: True(T) or False (F) a) Work could completely be converted into heat. b) c) The results of the zeroth, first and second laws of thermodynamics
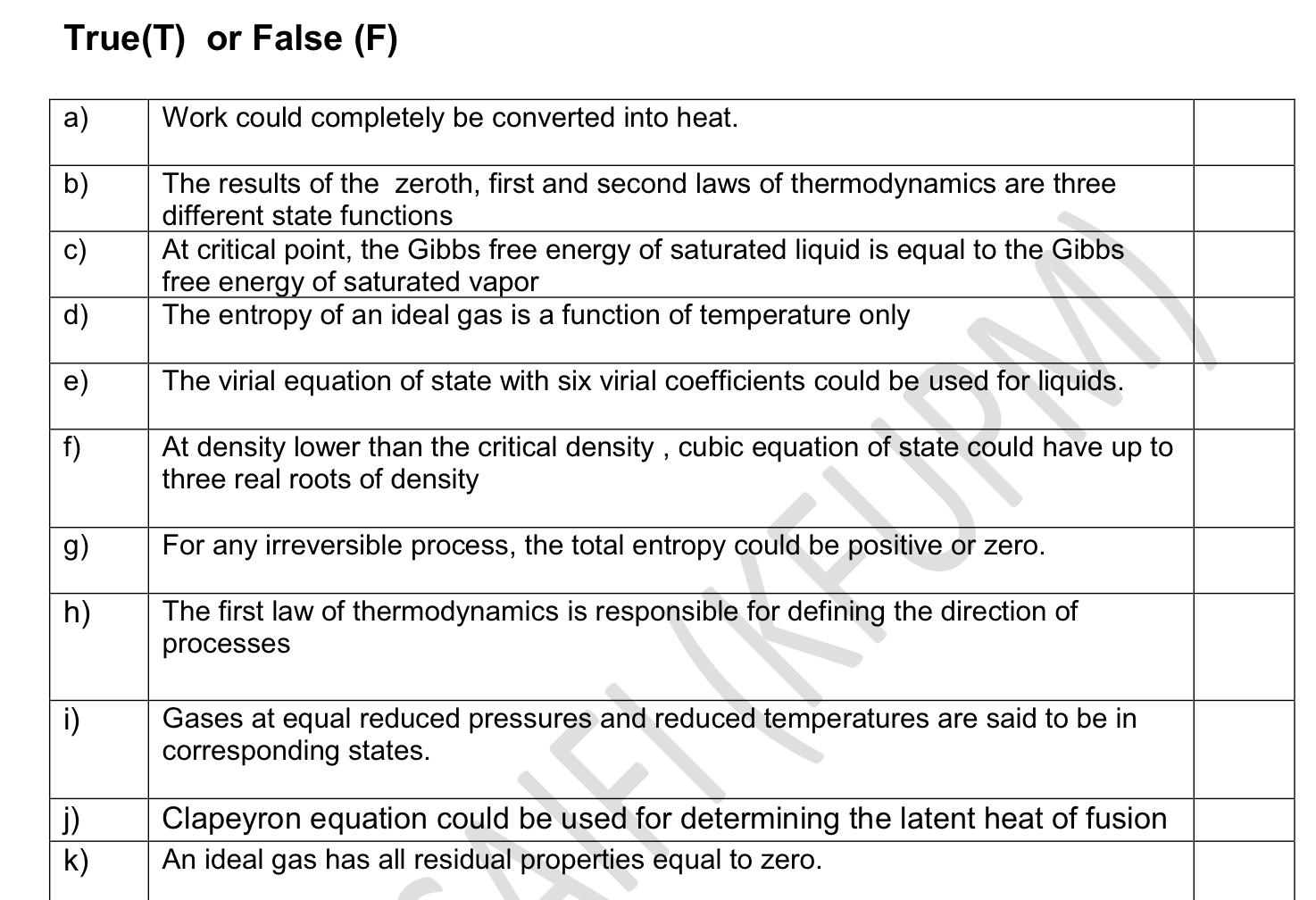
True(T) or False (F) a) Work could completely be converted into heat. b) c) The results of the zeroth, first and second laws of thermodynamics are three different state functions At critical point, the Gibbs free energy of saturated liquid is equal to the Gibbs free energy of saturated vapor The entropy of an ideal gas is a function of temperature only d) The virial equation of state with six virial coefficients could be used for liquids. f) At density lower than the critical density, cubic equation of state could have up to three real roots of density g) For any irreversible process, the total entropy could be positive or zero. h) The first law of thermodynamics is responsible for defining the direction of processes i) Gases at equal reduced pressures and reduced temperatures are said to be in corresponding states. de j) k) Clapeyron equation could be used for determining the latent heat of fusion An ideal gas has all residual properties equal to zero
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


