Question: Try Again Your answer is incorrect. - Row 1: name of compound: You must include the cation charge in the name when the metal can
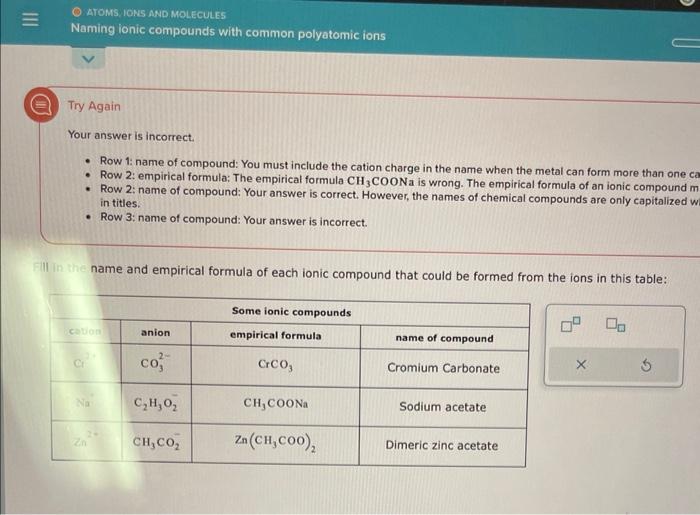
Try Again Your answer is incorrect. - Row 1: name of compound: You must include the cation charge in the name when the metal can form more than one c - Row 2: empirical formula: The empirical formula CH3COONa is wrong. The empirical formula of an ionic compound m - Row 2: name of compound: Your answer is correct. However, the names of chemical compounds are only capitalized v in titles. - Row 3: name of compound: Your answer is incorrect. name and empirical formula of each ionic compound that could be formed from the ions in this table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


