Question: Calculate the equilibrium constant from the standard free energy change. Using standard thermodynamie data (linked), calculate the equilibrium constant at 298.15 K for the
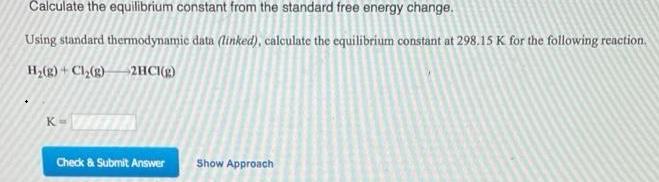
Calculate the equilibrium constant from the standard free energy change. Using standard thermodynamie data (linked), calculate the equilibrium constant at 298.15 K for the following reaction. H,(g) + Cl,(g)2HC(g) Check & Submit Answer Show Approach
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
6362793e73851_236795.pdf
180 KBs PDF File
6362793e73851_236795.docx
120 KBs Word File


