Question: Two 1-L beakers, A and B, each containing a different aqueous solution of manitol (a nonvolatlle sugar with MW = 182.17 a/mol) are placed together
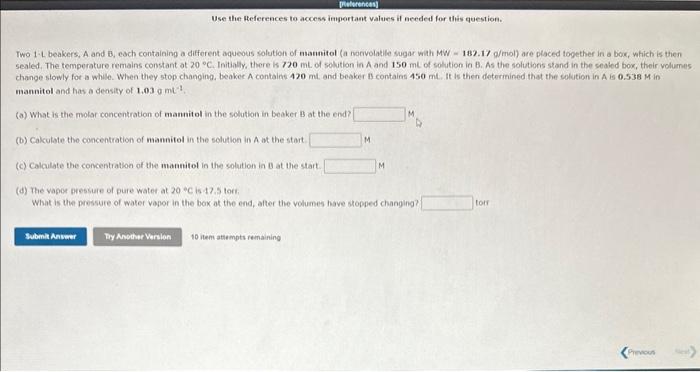
Two 1-L beakers, A and B, each containing a different aqueous solution of manitol (a nonvolatlle sugar with MW = 182.17 a/mol) are placed together in a bok, which is then sealed. The temperature remains constant at 20C. Initialily, there is 720mL of solution in A and 150mL of solution in B. As the solutions stand in the sealed box, their volumes change slowly for a while. When they stop changing, beakec A contains 420mt and beaker B contains 450mt. It is then determined that the solution in A is 0.538M in mannitol and has a deosity of 1,03mt1. (a) What is the molar concentration of manuitol in the solation in beaker B at the end? (b) Cakculate the concentration of mannitol in the solution in A at the start. (c) Calculate the concentration of the mantitol in the solution in 0 at the start. (d) The vapos pressure of pure water at 20C is 47.5 torr. What is the pressure of water vagor in the box at the end, after the volumes have stopped changing? 10 item attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


