Question: Two constant - volume gas thermometers are assembled, one with nitrogen and the other with hydrogen. Both contain enough gas so that p 3 =
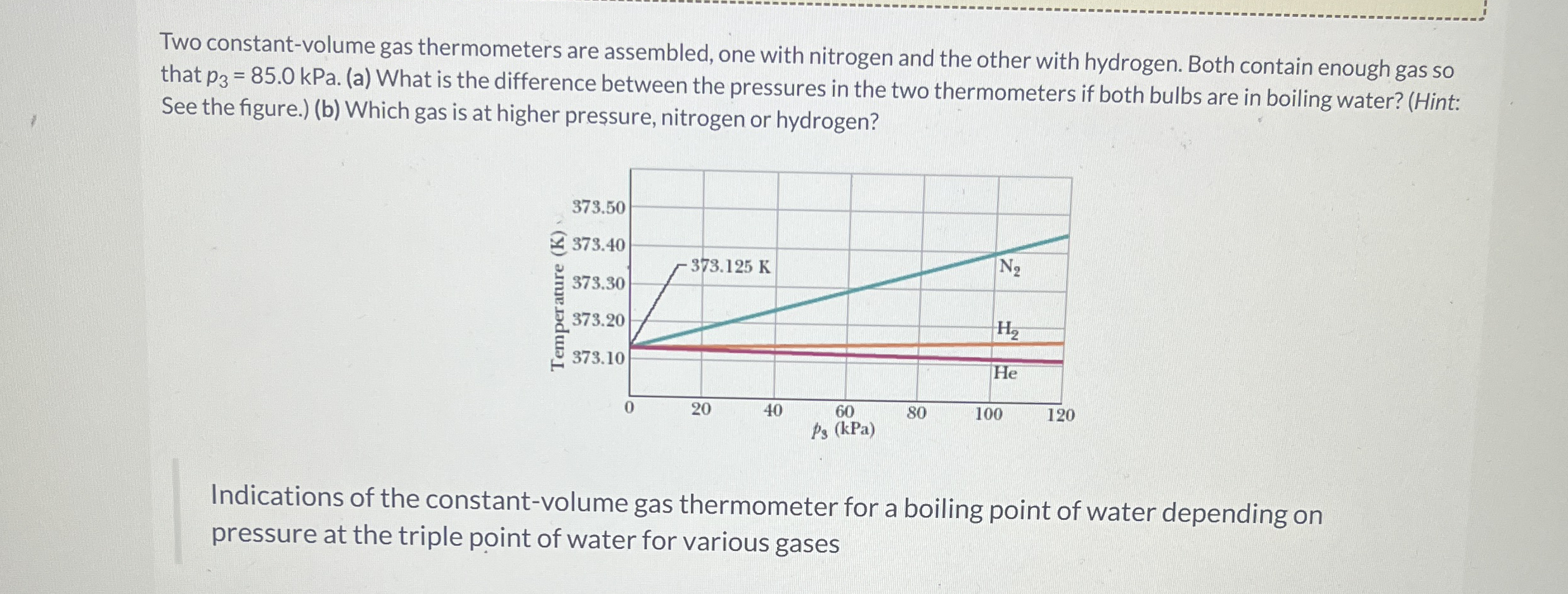
Two constantvolume gas thermometers are assembled, one with nitrogen and the other with hydrogen. Both contain enough gas so that kPa. a What is the difference between the pressures in the two thermometers if both bulbs are in boiling water? Hint: See the figure.b Which gas is at higher pressure, nitrogen or hydrogen?
Indications of the constantvolume gas thermometer for a boiling point of water depending on pressure at the triple point of water for various gases
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


