Question: Two elementary steps and their equilibrium constants are given below. Determine the value of the equilibrium constant for the overall reaction. A+BX K-5,274 X+B 2C+D
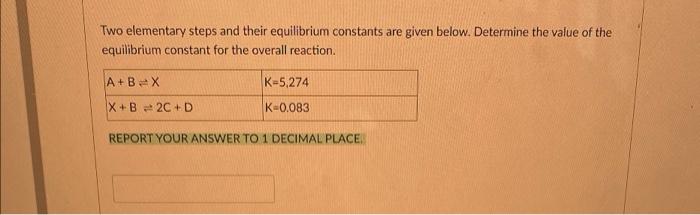
Two elementary steps and their equilibrium constants are given below. Determine the value of the equilibrium constant for the overall reaction. A+BX K-5,274 X+B 2C+D K-0.083 REPORT YOUR ANSWER TO 1 DECIMAL PLACE
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


