Question: Two solutions, initially at 23.49C, are mixed in a perfect coffee cup calorimeter at the same temperature. When 100.0mL of 0.186M sodium chloride is mixed
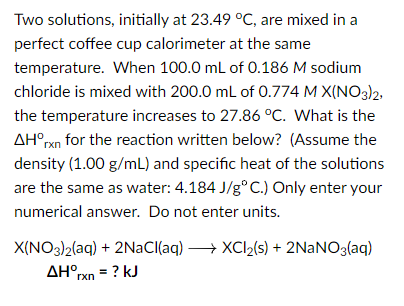
Two solutions, initially at 23.49C, are mixed in a perfect coffee cup calorimeter at the same temperature. When 100.0mL of 0.186M sodium chloride is mixed with 200.0mL of 0.774MX(NO3)2, the temperature increases to 27.86C. What is the Hrxn for the reaction written below? (Assume the density (1.00g/mL) and specific heat of the solutions are the same as water: 4.184J/gC.) Only enter your numerical answer. Do not enter units. X(NO3)2(aq)Hrxn+2NaCl(aq)XCl2(s)+2NaNO3(aq)=?kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


