Question: For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced. reaction highlighted atom is being...
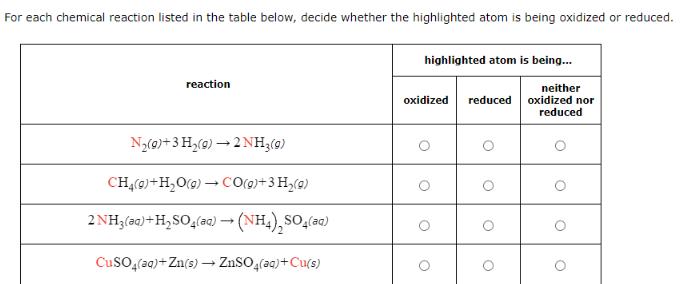
For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced. reaction highlighted atom is being... neither oxidized reduced oxidized nor reduced N2(0)+3H2(g) 2 NH3(g) CH4(g) +HO(g) CO(g)+3 H(g) -> 2NH3(aq) + H2SO4(aq) (NH4)2SO4(aq) CuSO4(aq)+Zn(s) ZnSO4(aq) + Cu(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


