Question: Explore a face-centered cubic unit cell. av2 Unit cell Cube face (3 of 6 We will now determine the percent of the unit cell
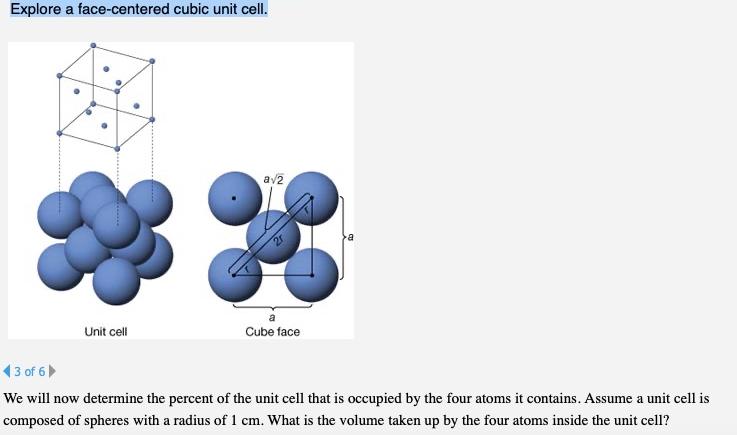
Explore a face-centered cubic unit cell. av2 Unit cell Cube face (3 of 6 We will now determine the percent of the unit cell that is occupied by the four atoms it contains. Assume a unit cell is composed of spheres with a radius of 1 cm. What is the volume taken up by the four atoms inside the unit cell?
Step by Step Solution
★★★★★
3.43 Rating (150 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


