Question: Upload answer sheets (i) Write the half-cell reaction, the net reaction and cell EMF of the following cell which operated at 75 C: Mn|Mn2+ (0.05
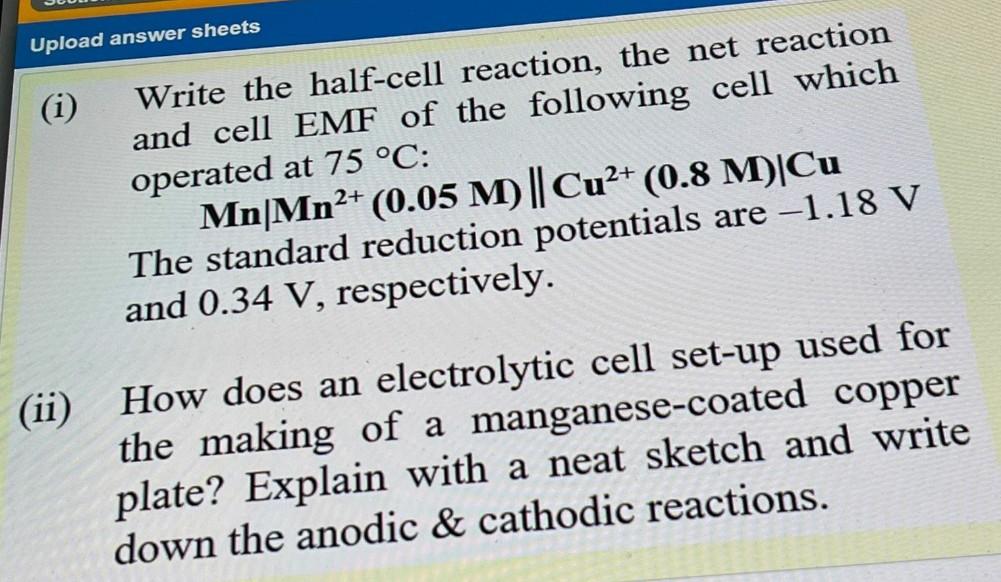
Upload answer sheets (i) Write the half-cell reaction, the net reaction and cell EMF of the following cell which operated at 75 C: Mn|Mn2+ (0.05 M) || Cu2+ (0.8 M)Cu The standard reduction potentials are -1.18 V and 0.34 V, respectively. (ii) How does an electrolytic cell set-up used for the making of a manganese-coated copper plate? Explain with a neat sketch and write down the anodic & cathodic reactions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


