Question: Upon completion of this learning module, you should be able to (1) use dimensional analysis to convert between various scientific units; (2) use sigma-t (t)
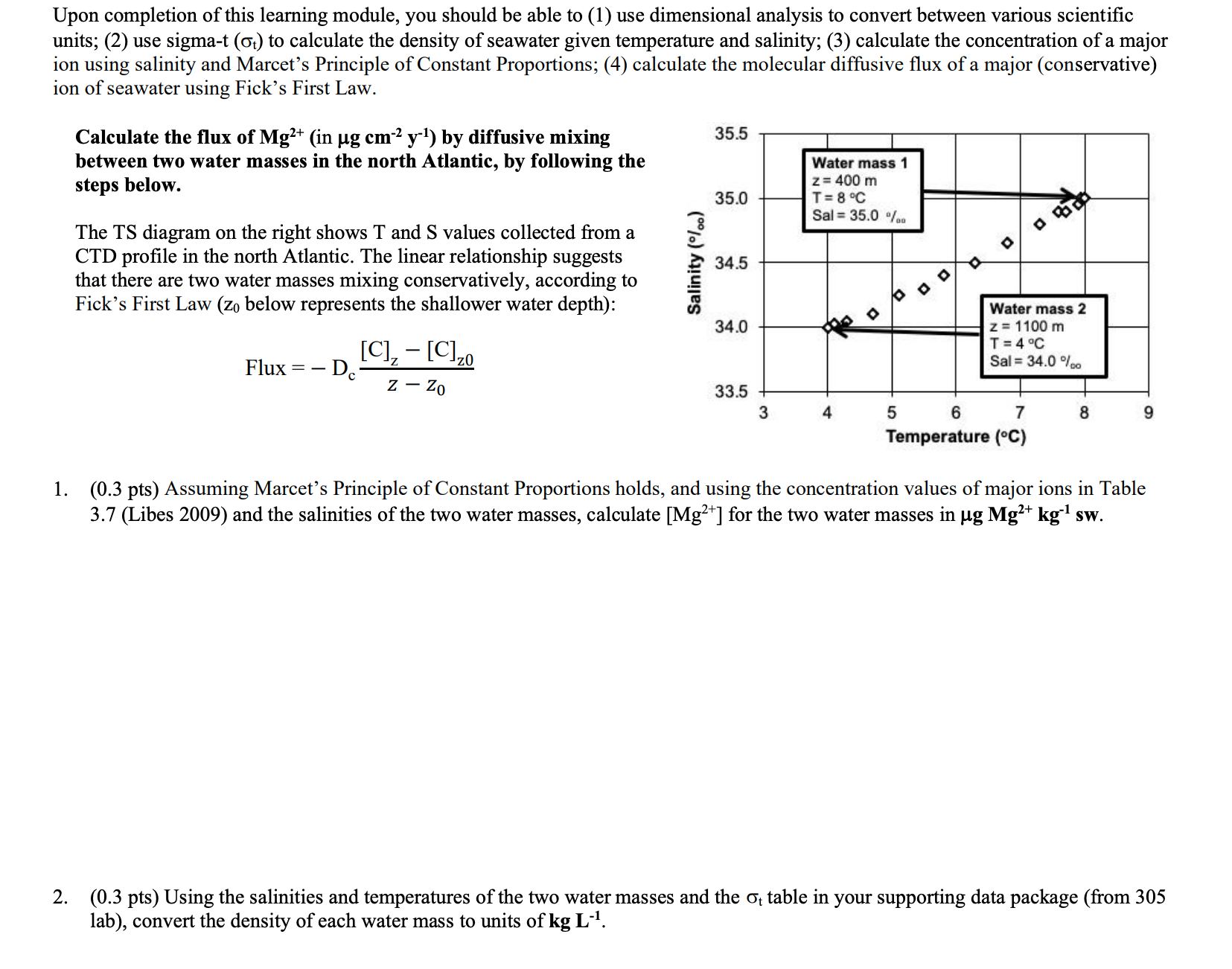

Upon completion of this learning module, you should be able to (1) use dimensional analysis to convert between various scientific units; (2) use sigma-t (t) to calculate the density of seawater given temperature and salinity; (3) calculate the concentration of a major ion using salinity and Marcet's Principle of Constant Proportions; (4) calculate the molecular diffusive flux of a major (conservative) ion of seawater using Fick's First Law. Calculate the flux of Mg2+ (in gcm2y1 ) by diffusive mixing between two water masses in the north Atlantic, by following the steps below. The TS diagram on the right shows T and S values collected from a CTD profile in the north Atlantic. The linear relationship suggests that there are two water masses mixing conservatively, according to Fick's First Law ( z0 below represents the shallower water depth): Flux=Dczz0[C]z[C]z0 1. (0.3 pts) Assuming Marcet's Principle of Constant Proportions holds, and using the concentration values of major ions in Table 3.7 (Libes 2009) and the salinities of the two water masses, calculate [Mg2+] for the two water masses in gMg2+kg1sw. 2. (0.3 pts) Using the salinities and temperatures of the two water masses and the t table in your supporting data package (from 305 lab), convert the density of each water mass to units of kgLL1. 3. (0.6 pts) Using your answers in 2 , convert the [Mg2+] you calculated in 1 for the two water masses to units of gMg2+cm3sw. 4. (0.3 pts) Calculate the Mg2+ concentration gradient, zz0[Mg2+]z[Mg2+]z, in units of gMg2+cm4. 5. (0.5 pts) Complete the flux calculation using a diffusion coefficient of Mg2+,Dc=5106cm2s1. Remember to report your answer in gMg2+cm2y1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


