Question: Uranium in ore exist in + 4 oxidation states which during sulfuric acid leching is oxidized to + 6 oxidation state as uranyl ion (
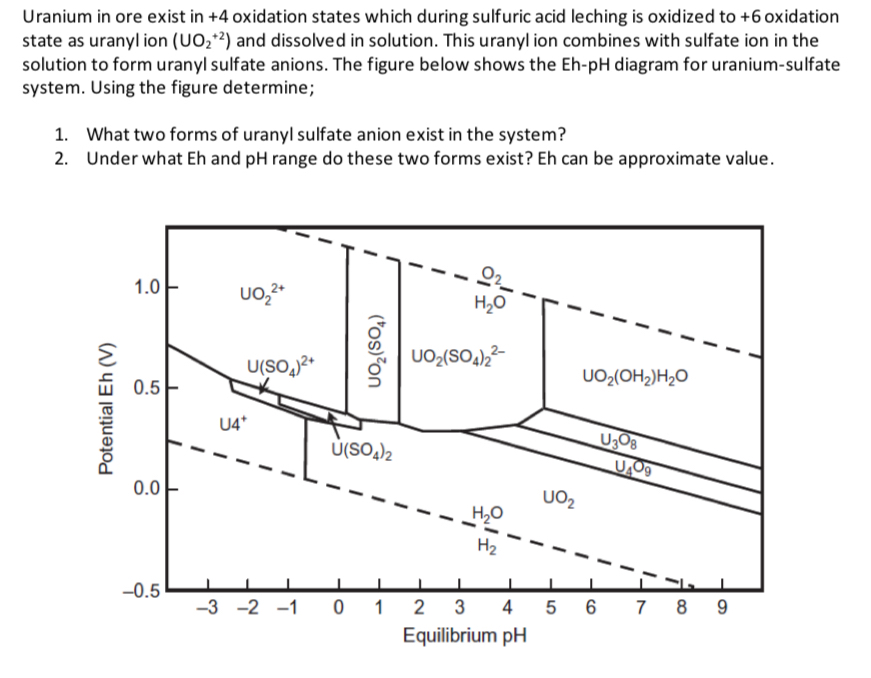
Uranium in ore exist in oxidation states which during sulfuric acid leching is oxidized to oxidation state as uranyl ion and dissolved in solution. This uranyl ion combines with sulfate ion in the solution to form uranyl sulfate anions. The figure below shows the Eh diagram for uraniumsulfate system. Using the figure determine;
What two forms of uranyl sulfate anion exist in the system?
Under what Eh and pH range do these two forms exist? Eh can be approximate value.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


