Question: URGENT now!! PLS STEP BY STEP!!!!! Acetone (A) MIC with 50% acetone by weight in a continuous and countercurrent extraction system Acetone is extracted from
URGENT now!! PLS STEP BY STEP!!!!!
Acetone (A) MIC with 50% acetone by weight in a continuous and countercurrent extraction system Acetone is extracted from the mixture (methyl isobutyl ketone) (B) using water. Process, The amount of acetone retained by 1 kg of solvent in the obtained extract phase is the amount of acetone that 1 kg of solvent can hold. It is carried out to be 70% of the maximum amount of acetone. Operation The slope of the line is determined as 0.65. Mixture of methyl isobutyl ketone (MIK) and water Assuming that it does not dissolve at all, find the number of theoretical equilibrium steps required for the process.
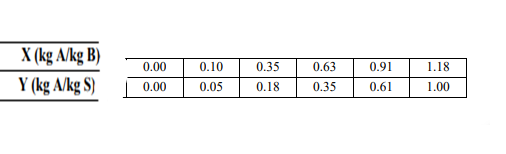
Y(kgA/kgS)X(kgA/kgB) \begin{tabular}{|l|l|l|l|l|l|} \hline 0.00 & 0.10 & 0.35 & 0.63 & 0.91 & 1.18 \\ \hline 0.00 & 0.05 & 0.18 & 0.35 & 0.61 & 1.00 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


