Question: Use a spreadsheet and construct curves for the following titrations. Calculate potentials after the addition of 10.00, 25.00, 49.00, 49.90, 50.00, 50.10, 51.00, and 60.00
Use a spreadsheet and construct curves for the following titrations. Calculate potentials after the addition of 10.00, 25.00, 49.00, 49.90, 50.00, 50.10, 51.00, and 60.00 mL of the reagent. Where necessary, assume that [H+]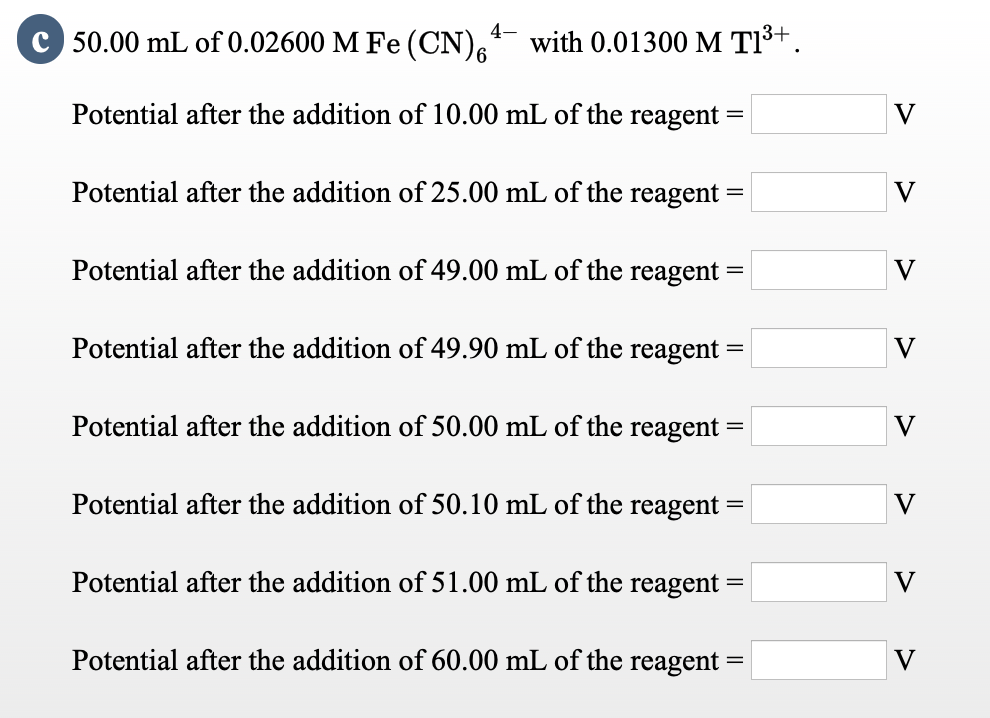 ?
?
= 1.00 throughout.
4- C 50.00 mL of 0.02600 M Fe (CN)6 with 0.01300 M T1+. Potential after the addition of 10.00 mL of the reagent Potential after the addition of 25.00 mL of the reagent = Potential after the addition of 49.00 mL of the reagent Potential after the addition of 49.90 mL of the reagent Potential after the addition of 50.00 mL of the reagent Potential after the addition of 50.10 mL of the reagent = Potential after the addition of 60.00 mL of the reagent = = = = Potential after the addition of 51.00 mL of the reagent: = V V
Step by Step Solution
3.33 Rating (171 Votes )
There are 3 Steps involved in it
03 5... View full answer

Get step-by-step solutions from verified subject matter experts


