Question: Use balances and not guesses based on stoichiometry. A double effect evaporator (consisting of two evaporators connected in series) is used to obtain fresh water

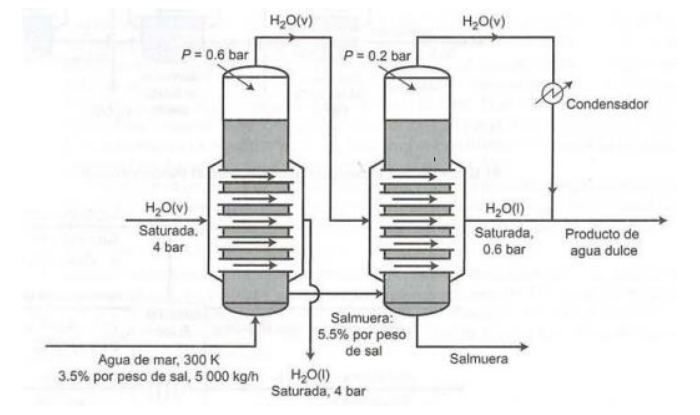


Use balances and not guesses based on stoichiometry. A double effect evaporator (consisting of two evaporators connected in series) is used to obtain fresh water from seawater containing 3.5% by weight of dissolved salts. The process flow chart is shown below. H2O(v) H2O(v) P = 0.6 bar P=0.2 bar Condensador H.O(v) Saturada. 4 bar H2O(1) Saturada, 0.6 bar Producto de agua dulce Salmuera: 5.5% por peso de sal H2O(1) Saturada, 4 bar Agua de mar, 300 K 3.5% por peso de sal, 5 000 kg/h Salmuera Seawater enters the first effect at 300 K at a rate of 5000 kg/h, and saturated steam at 4 bar is fed into a bank of tubes in the first effect. The steam is condensed at 4 bar, and the condensate is withdrawn at the saturation temperature that corresponds to this pressure. The heat given off by the steam that condenses on the tubes causes the water to evaporate from the brine solution at a pressure of 0.6 bar maintained in the effect. The outlet brine contains 5.5% by weight of salt. The steam generated in the first effect is fed to a bank of tubes in the second effect. The condensate coming from bank and the steam generated in the second effect at a pressure of 0.2 bar constitute the fresh water that is produced in the process. a) At what rate should steam be fed to the first effect? b) What is the rate of production of fresh water? What is the salt concentration (weight fraction) of the final brine solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


