Question: use defractometer to predict the cubic metal element Lattice Reflection present Reflection absent Simple cubic BCC FCC Every (hkl) plane 1h + k + l)
use defractometer to predict the cubic metal element
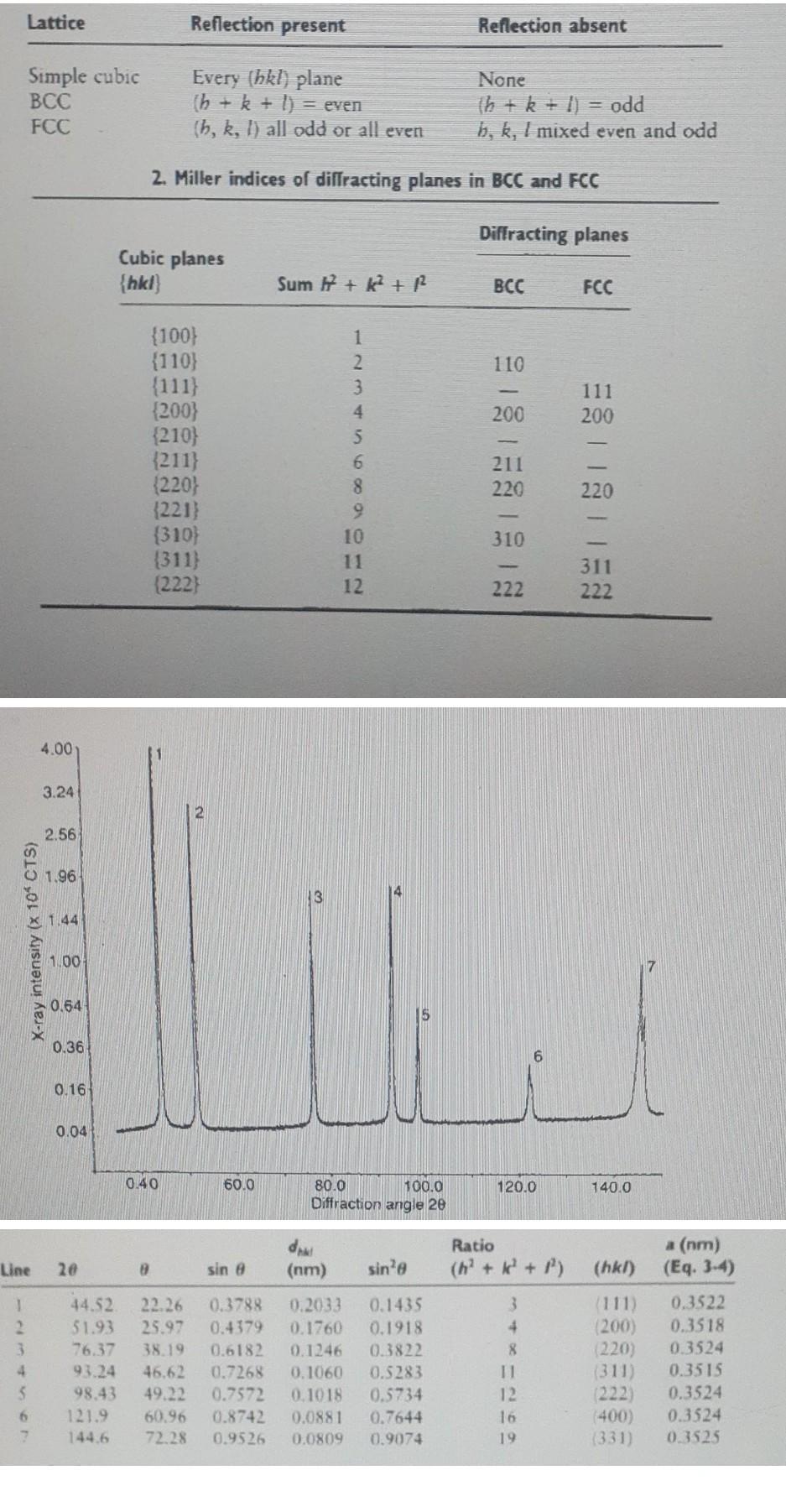
Lattice Reflection present Reflection absent Simple cubic BCC FCC Every (hkl) plane 1h + k + l) = even (h, k, l) all odd or all even None Th + k + 1) = odd b, k, I mixed even and odd 2. Miller indices of diffracting planes in BCC and FCC Diffracting planes Cubic planes {hkl) Sum + k + R BCC FCC 110 111 200 200 {100} {110} {111) {200} (210) {211} (220) (221) (310) (311) (222) 1 2 3 4 S 6 8 9 10 11 12 211 220 220 310 311 222 222 4.00 3.24 2.56 1.96 4 x 1.44 X-ray intensity (x 10* CTS) 1.00 0.64 0.36 0.16 0.04 0.40 60.0 120.0 140.0 80.0 100.0 Diffraction angle 20 du (nm) Ratio (h + k + ) a (nm) (Eq. 3-4) Line 28 8 sin e sine (hk) 1 3 4 5 6 2 44.52 51.93 76.37 93.24 98.43 121.9 144.6 22.26 25.97 38.19 46.62 49.22 60.96 72.28 0.3788 0.4379 0.6182 0.7268 0.7572 0.8742 0.9526 0.2033 0.1760 0.1246 0.1060 0.1018 0.0881 0.0809 0.1435 0.1918 0.3822 0.5283 0.5734 0.7644 0.9074 3 4 8 11 12 16 19 (111) (200) (220) (311) (222) 400) (331) 0.3522 0.3518 0.3524 0.3515 0.3524 0.3524 0.3525
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


